Enhanced immunity to Plasmodium falciparum circumsporozoite protein (PfCSP) by using Salmonella enterica serovar Typhi expressing PfCSP and a PfCSP-encoding DNA vaccine in a heterologous prime-boost strategy
- PMID: 17502396
- PMCID: PMC1951980
- DOI: 10.1128/IAI.00356-07
Enhanced immunity to Plasmodium falciparum circumsporozoite protein (PfCSP) by using Salmonella enterica serovar Typhi expressing PfCSP and a PfCSP-encoding DNA vaccine in a heterologous prime-boost strategy
Abstract
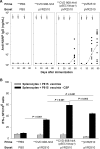
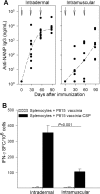
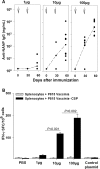
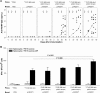
Two Salmonella enterica serovar Typhi strains that express and export a truncated version of Plasmodium falciparum circumsporozoite surface protein (tCSP) fused to Salmonella serovar Typhi cytolysin A (ClyA) were constructed as a first step in the development of a preerythrocytic malaria vaccine. Synthetic codon-optimized genes (t-csp1 and t-csp2), containing immunodominant B- and T-cell epitopes present in native P. falciparum circumsporozoite surface protein (PfCSP), were fused in frame to the carboxyl terminus of the ClyA gene (clyA::t-csp) in genetically stabilized expression plasmids. Expression and export of ClyA-tCSP1 and ClyA-tCSP2 by Salmonella serovar Typhi vaccine strain CVD 908-htrA were demonstrated by immunoblotting of whole-cell lysates and culture supernatants. The immunogenicity of these constructs was evaluated using a "heterologous prime-boost" approach consisting of mucosal priming with Salmonella serovar Typhi expressing ClyA-tCSP1 and ClyA-tCSP2, followed by parenteral boosting with PfCSP DNA vaccines pVR2510 and pVR2571. Mice primed intranasally on days 0 and 28 with CVD 908-htrA(pSEC10tcsp2) and boosted intradermally on day 56 with PfCSP DNA vaccine pVR2571 induced high titers of serum NANP immunoglobulin G (IgG) (predominantly IgG2a); no serological responses to DNA vaccination were observed in the absence of Salmonella serovar Typhi-PfCSP priming. Mice primed with Salmonella serovar Typhi expressing tCSP2 and boosted with PfCSP DNA also developed high frequencies of gamma interferon-secreting cells, which surpassed those produced by PfCSP DNA in the absence of priming. A prime-boost regimen consisting of mucosal delivery of PfCSP exported from a Salmonella-based live-vector vaccine followed by a parenteral PfCSP DNA boosting is a promising strategy for the development of a live-vector-based malaria vaccine.
Figures

Similar articles
-
Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA.Infect Immun. 2004 Dec;72(12):7096-106. doi: 10.1128/IAI.72.12.7096-7106.2004. Infect Immun. 2004. PMID: 15557633 Free PMC article.
-
Liver-Directed AAV8 Booster Vaccine Expressing Plasmodium falciparum Antigen Following Adenovirus Vaccine Priming Elicits Sterile Protection in a Murine Model.Front Immunol. 2021 Jun 23;12:612910. doi: 10.3389/fimmu.2021.612910. eCollection 2021. Front Immunol. 2021. PMID: 34248928 Free PMC article.
-
Expression of the Plasmodium falciparum immunodominant epitope (NANP)(4) on the surface of Salmonella enterica using the autotransporter MisL.Infect Immun. 2002 Jul;70(7):3611-20. doi: 10.1128/IAI.70.7.3611-3620.2002. Infect Immun. 2002. PMID: 12065502 Free PMC article.
-
Prime-boost vectored malaria vaccines: progress and prospects.Hum Vaccin. 2010 Jan;6(1):78-83. doi: 10.4161/hv.6.1.10116. Epub 2010 Jan 18. Hum Vaccin. 2010. PMID: 20061802 Review.
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
Cited by
-
Lactobacillus plantarum producing a Chlamydia trachomatis antigen induces a specific IgA response after mucosal booster immunization.PLoS One. 2017 May 3;12(5):e0176401. doi: 10.1371/journal.pone.0176401. eCollection 2017. PLoS One. 2017. PMID: 28467432 Free PMC article.
-
Live attenuated rubella vectors expressing Plasmodium falciparum circumsporozoite protein (Pf-CSP) provide a novel malaria vaccine platform in the rhesus macaque.Biochem Biophys Res Commun. 2021 Nov 5;577:58-63. doi: 10.1016/j.bbrc.2021.08.052. Epub 2021 Aug 27. Biochem Biophys Res Commun. 2021. PMID: 34507066 Free PMC article.
-
Protective antibody and CD8+ T-cell responses to the Plasmodium falciparum circumsporozoite protein induced by a nanoparticle vaccine.PLoS One. 2012;7(10):e48304. doi: 10.1371/journal.pone.0048304. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144750 Free PMC article.
-
Use of mchI encoding immunity to the antimicrobial peptide microcin H47 as a plasmid selection marker in attenuated bacterial live vectors.Infect Immun. 2008 Oct;76(10):4422-30. doi: 10.1128/IAI.00487-08. Epub 2008 Jul 28. Infect Immun. 2008. PMID: 18663003 Free PMC article.
-
Advances in the development of Salmonella-based vaccine strategies for protection against Salmonellosis in humans.J Appl Microbiol. 2021 Dec;131(6):2640-2658. doi: 10.1111/jam.15055. Epub 2021 Apr 3. J Appl Microbiol. 2021. PMID: 33665941 Free PMC article. Review.
References
-
- Aguiar, J. C., R. C. Hedstrom, W. O. Rogers, Y. Charoenvit, J. B. Sacci, Jr., D. E. Lanar, V. F. Majam, R. R. Stout, and S. L. Hoffman. 2001. Enhancement of the immune response in rabbits to a malaria DNA vaccine by immunization with a needle-free jet device. Vaccine 20:275-280. - PubMed
-
- Aidoo, M., A. Lalvani, C. E. Allsopp, M. Plebanski, S. J. Meisner, P. Krausa, M. Browning, S. Morris-Jones, F. Gotch, D. A. Fidock, M. Takiguchi, K. J. H. Robson, B. M. Greenwood, P. Druilhe, H. C. Whittle, and A. V. Hill. 1995. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet 345:1003-1007. - PubMed
-
- Aidoo, M., and V. Udhayakumar. 2000. Field studies of cytotoxic T lymphocytes in malaria infections: implications for malaria vaccine development. Parasitol. Today 16:50-56. - PubMed
-
- Alloueche, A., P. Milligan, D. J. Conway, M. Pinder, K. Bojang, T. Doherty, N. Tornieporth, J. Cohen, and B. M. Greenwood. 2003. Protective efficacy of the RTS,S/AS02 Plasmodium falciparum malaria vaccine is not strain specific. Am. J. Trop. Med. Hyg. 68:97-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

