Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus
- PMID: 17502406
- PMCID: PMC1932546
- DOI: 10.1128/AAC.00209-07
Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus
Abstract
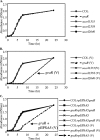
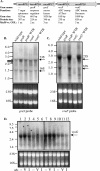
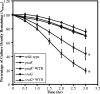
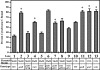
Current treatment for serious infections caused by methicillin-resistant Staphylococcus aureus relies heavily upon the glycopeptide antibiotic vancomycin. Unfortunately, this practice has led to an intermediate resistance phenotype that is particularly difficult to treat in invasive staphylococcal diseases, such as septicemia and its metastatic complications, including endocarditis. Although the vancomycin-intermediate resistance phenotype has been linked to abnormal cell wall structures and autolytic rates, the corresponding genetic changes have not been fully elucidated. Previously, whole-genome array studies listed numerous genes that are overexpressed in vancomycin-intermediate sensitive strains, including graRS (SACOL0716 to -0717), encoding a two-component regulatory system (TCRS), as well as the adjacent vraFG (SACOL0718 to -0720), encoding an ATP-binding cassette (ABC) transporter; but the exact contribution of these genes to increased vancomycin resistance has not been defined. In this study, we showed that isogenic strains with mutations in genes encoding the GraRS TCRS and the VraFG ABC transporter are hypersensitive to vancomycin as well as polymyxin B. Moreover, GraRS regulates the expression of the adjacent VraFG pump, reminiscent of gram-positive bacteriocin-immunity regulons. Mutations of graRS and vraFG also led to increased autolytic rates and a more negative net surface charge, which may explain, in part, to their increased sensitivity to cationic antimicrobial peptides. Taken together, these data reveal an important genetic mediator to the vancomycin-intermediate S. aureus phenotype and may hold clues to the selective pressures on staphylococci upon exposure to selective cationic peptide antibiotics used in clinical practice.
Figures
Similar articles
-
An induced mutation of ABC-transporter component VraF(K84E) contributes to vancomycin resistance and virulence in Staphylococcus aureus strain MW2.Int J Med Microbiol. 2024 Jun;315:151624. doi: 10.1016/j.ijmm.2024.151624. Epub 2024 Jun 2. Int J Med Microbiol. 2024. PMID: 38838390
-
The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides.Infect Immun. 2012 Jan;80(1):74-81. doi: 10.1128/IAI.05669-11. Epub 2011 Oct 10. Infect Immun. 2012. PMID: 21986630 Free PMC article.
-
GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus.Antimicrob Agents Chemother. 2012 Feb;56(2):1047-58. doi: 10.1128/AAC.05054-11. Epub 2011 Nov 28. Antimicrob Agents Chemother. 2012. PMID: 22123691 Free PMC article.
-
Adaptation of methicillin-resistant Staphylococcus aureus in the face of vancomycin therapy.Clin Infect Dis. 2006 Jan 1;42 Suppl 1:S40-50. doi: 10.1086/491713. Clin Infect Dis. 2006. PMID: 16323119 Review.
-
Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications.Clin Microbiol Rev. 2010 Jan;23(1):99-139. doi: 10.1128/CMR.00042-09. Clin Microbiol Rev. 2010. PMID: 20065327 Free PMC article. Review.
Cited by
-
Proteomics and Transcriptomics Uncover Key Processes for Elasnin Tolerance in Methicillin-Resistant Staphylococcus aureus.mSystems. 2022 Feb 22;7(1):e0139321. doi: 10.1128/msystems.01393-21. Epub 2022 Jan 25. mSystems. 2022. PMID: 35076266 Free PMC article.
-
Bacterial resistance mechanisms against host defense peptides.Cell Mol Life Sci. 2011 Jul;68(13):2243-54. doi: 10.1007/s00018-011-0716-4. Epub 2011 May 11. Cell Mol Life Sci. 2011. PMID: 21560069 Free PMC article. Review.
-
Molecular Basis of Non-β-Lactam Antibiotics Resistance in Staphylococcus aureus.Antibiotics (Basel). 2022 Oct 8;11(10):1378. doi: 10.3390/antibiotics11101378. Antibiotics (Basel). 2022. PMID: 36290036 Free PMC article. Review.
-
Colistin Induces S. aureus Susceptibility to Bacitracin.Front Microbiol. 2018 Nov 20;9:2805. doi: 10.3389/fmicb.2018.02805. eCollection 2018. Front Microbiol. 2018. PMID: 30515145 Free PMC article.
-
Environment Shapes the Accessible Daptomycin Resistance Mechanisms in Enterococcus faecium.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00790-19. doi: 10.1128/AAC.00790-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31332078 Free PMC article.
References
-
- Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122:461-472. - PubMed
-
- Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

