Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3
- PMID: 17502423
- PMCID: PMC2064207
- DOI: 10.1083/jcb.200611063
Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3
Abstract
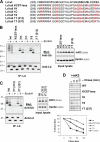
The mechanism by which substrates for endoplasmic reticulum-associated degradation are retrotranslocated to the cytosol remains largely unknown, although ubiquitination is known to play a key role. The mouse gamma-herpesvirus protein mK3 is a viral RING-CH-type E3 ligase that specifically targets nascent major histocompatibility complex I heavy chain (HC) for degradation, thus blocking the immune detection of virus-infected cells. To address the question of how HC is retrotranslocated and what role mK3 ligase plays in this action, we investigated ubiquitin conjugation sites on HC using mutagenesis and biochemistry approaches. In total, our data demonstrate that mK3-mediated ubiquitination can occur via serine, threonine, or lysine residues on the HC tail, each of which is sufficient to induce the rapid degradation of HC. Given that mK3 has numerous cellular and viral homologues, it will be of considerable interest to determine the pervasiveness of this novel mechanism of ubiquitination.
Figures






References
-
- Baker, R.T. 1996. Protein expression using ubiquitin fusion and cleavage. Curr. Opin. Biotechnol. 7:541–546. - PubMed
-
- Barel, M.T., N. Pizzato, D. van Leeuwen, P.L. Bouteiller, E.J. Wiertz, and F. Lenfant. 2003. Amino acid composition of alpha1/alpha2 domains and cytoplasmic tail of MHC class I molecules determine their susceptibility to human cytomegalovirus US11-mediated down-regulation. Eur. J. Immunol. 33:1707–1716. - PubMed
-
- Bhamidipati, A., V. Denic, E.M. Quan, and J.S. Weissman. 2005. Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol. Cell. 19:741–751. - PubMed
-
- Biederer, T., C. Volkwein, and T. Sommer. 1997. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 278:1806–1809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

