Trans-SNARE complex assembly and yeast vacuole membrane fusion
- PMID: 17502611
- PMCID: PMC1885575
- DOI: 10.1073/pnas.0702290104
Trans-SNARE complex assembly and yeast vacuole membrane fusion
Abstract
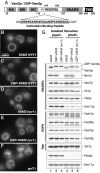
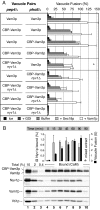
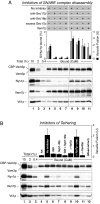
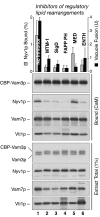
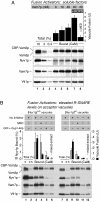
cis-SNARE complexes (anchored in one membrane) are disassembled by Sec17p (alpha-SNAP) and Sec18p (NSF), permitting the unpaired SNAREs to assemble in trans. We now report a direct assay of trans-SNARE complex formation during yeast vacuole docking. SNARE complex assembly and fusion is promoted by high concentrations of the SNARE Vam7p or Nyv1p or by addition of HOPS (homotypic fusion and vacuole protein sorting), a Ypt7p (Rab)-effector complex with a Sec1/Munc18-family subunit. Inhibitors that target Ypt7p, HOPS, or key regulatory lipids prevent trans-SNARE complex assembly and ensuing fusion. Strikingly, the lipid ligand MED (myristoylated alanine-rich C kinase substrate effector domain) or elevated concentrations of Sec17p, which can displace HOPS from SNARE complexes, permit full trans-SNARE pairing but block fusion. These findings suggest that efficient fusion requires trans-SNARE complex associations with factors such as HOPS and subsequent regulated lipid rearrangements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion.EMBO J. 2005 May 18;24(10):1775-86. doi: 10.1038/sj.emboj.7600658. Epub 2005 May 5. EMBO J. 2005. PMID: 15889152 Free PMC article.
-
Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion.EMBO J. 2007 Dec 12;26(24):4935-45. doi: 10.1038/sj.emboj.7601915. Epub 2007 Nov 15. EMBO J. 2007. PMID: 18007597 Free PMC article.
-
HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly.Mol Biol Cell. 2010 Jul 1;21(13):2297-305. doi: 10.1091/mbc.e10-01-0044. Epub 2010 May 12. Mol Biol Cell. 2010. PMID: 20462954 Free PMC article.
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
-
Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms.Annu Rev Biochem. 2000;69:247-75. doi: 10.1146/annurev.biochem.69.1.247. Annu Rev Biochem. 2000. PMID: 10966459 Review.
Cited by
-
Munc18 and Munc13 serve as a functional template to orchestrate neuronal SNARE complex assembly.Nat Commun. 2019 Jan 8;10(1):69. doi: 10.1038/s41467-018-08028-6. Nat Commun. 2019. PMID: 30622273 Free PMC article.
-
Excess vacuolar SNAREs drive lysis and Rab bypass fusion.Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13551-8. doi: 10.1073/pnas.0704741104. Epub 2007 Aug 15. Proc Natl Acad Sci U S A. 2007. PMID: 17699614 Free PMC article.
-
Membrane fusion.Nat Struct Mol Biol. 2008 Jul;15(7):658-64. doi: 10.1038/nsmb.1451. Nat Struct Mol Biol. 2008. PMID: 18618939 Free PMC article. Review.
-
Vacuolar SNARE protein transmembrane domains serve as nonspecific membrane anchors with unequal roles in lipid mixing.J Biol Chem. 2015 May 15;290(20):12821-32. doi: 10.1074/jbc.M115.647776. Epub 2015 Mar 27. J Biol Chem. 2015. PMID: 25817997 Free PMC article.
-
SNAREs support atlastin-mediated homotypic ER fusion in Saccharomyces cerevisiae.J Cell Biol. 2015 Aug 3;210(3):451-70. doi: 10.1083/jcb.201501043. Epub 2015 Jul 27. J Cell Biol. 2015. PMID: 26216899 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

