In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds
- PMID: 17502613
- PMCID: PMC1885572
- DOI: 10.1073/pnas.0701829104
In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds
Abstract
The goal of this study was to create an in vitro cell culture system that captures essential features of the in vivo erythroid micronucleus (MN) genotoxicity assay, thus enabling increased throughput and controlled studies of the hematopoietic DNA damage response. We show that adult bone marrow (BM) cultures respond to erythropoietin, the principal hormone that stimulates erythropoiesis, with physiological erythropoietic proliferation, differentiation, and enucleation. We then show that this in vitro erythropoietic system clearly signals exposure to genotoxicants through erythroid MN formation. Furthermore, we determined that DNA repair-deficient (MGMT(-/-)) BM displayed sensitivity to genotoxic exposure in vivo compared with WT BM and that this phenotypic response was reflected in erythropoietic cultures. These findings suggest that this in vitro erythroid MN assay is capable of screening for genotoxicity on BM in a physiologically reflective manner. Finally, responses to genotoxicants during erythroid differentiation varied with exposure time, demonstrating that this system can be used to study the effect of DNA damage at specific developmental stages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
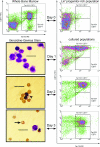


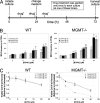
References
-
- Zon LI. Hematopoiesis. New York: Oxford Univ Press; 2001.
-
- Lodish HF. Molecular Cell Biology. New York: Freeman; 2003.
-
- Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys P, MacGregor JT. Environ Mol Mutagen. 1991;18:277–291. - PubMed
-
- Roth RB, Samson LD. Mutat Res. 2000;462:107–120. - PubMed
-
- Cai S, Hartwell JR, Cooper RJ, Juliar BE, Kreklau E, Abonour R, Goebel WS, Pollok KE. Mol Ther. 2006;13:1016–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

