beta-Actin regulates platelet nitric oxide synthase 3 activity through interaction with heat shock protein 90
- PMID: 17502619
- PMCID: PMC1885589
- DOI: 10.1073/pnas.0611416104
beta-Actin regulates platelet nitric oxide synthase 3 activity through interaction with heat shock protein 90
Abstract
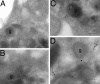
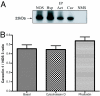
Cytoskeletal proteins are crucial in maintaining cellular structure and, in certain cell types, also play an essential role in motility and shape change. Nitric oxide (NO) is an important paracrine mediator of vascular and platelet function and is produced in the vasculature by the enzyme NO synthase type 3 (NOS-3). Here, we demonstrate in human platelets that the polymerization state of beta-actin crucially regulates the activation state of NOS-3, and hence NO formation, through altering its binding of heat shock protein 90 (Hsp90). We found that NOS-3 binds to the globular, but not the filamentous, form of beta-actin, and the affinity of NOS-3 for globular beta-actin is, in turn, increased by Hsp90. Formation of this ternary complex among NOS-3, globular beta-actin, and Hsp90, in turn, results in an increase in both NOS activity and cyclic guanosine-3',5'-monophosphate, an index of bioactive NO, as well as an increased rate of Hsp90 degradation, thus limiting the duration for which NOS-3 remains activated. These observations suggest that beta-actin plays a critical role in regulating NO formation and signaling in platelets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

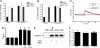


Similar articles
-
Beta-actin: a regulator of NOS-3.Sci STKE. 2007 Sep 18;2007(404):pe52. doi: 10.1126/stke.4042007pe52. Sci STKE. 2007. PMID: 17878410 Review.
-
Proteolytic degradation of nitric oxide synthase isoforms by calpain is modulated by the expression levels of HSP90.FEBS J. 2007 Dec;274(23):6116-27. doi: 10.1111/j.1742-4658.2007.06133.x. Epub 2007 Oct 29. FEBS J. 2007. PMID: 17970747
-
Pyridoxine improves platelet nitric oxide synthase dysfunction induced by advanced glycation end products in vitro.Int J Vitam Nutr Res. 2010 Jun;80(3):168-77. doi: 10.1024/0300-9831/a000019. Int J Vitam Nutr Res. 2010. PMID: 21234858
-
Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase.Circulation. 2005 Jun 28;111(25):3473-80. doi: 10.1161/CIRCULATIONAHA.105.546812. Epub 2005 Jun 20. Circulation. 2005. PMID: 15967841
-
Regulation of endothelial nitric oxide synthase activity by protein-protein interaction.Curr Pharm Des. 2014;20(22):3514-20. doi: 10.2174/13816128113196660752. Curr Pharm Des. 2014. PMID: 24180383 Free PMC article. Review.
Cited by
-
Bidirectional cross-regulation between the endothelial nitric oxide synthase and β-catenin signalling pathways.Cardiovasc Res. 2014 Oct 1;104(1):116-26. doi: 10.1093/cvr/cvu173. Epub 2014 Jul 25. Cardiovasc Res. 2014. PMID: 25062958 Free PMC article.
-
Platelet redox balance in diabetic patients with hypertension improved by n-3 fatty acids.Diabetes Care. 2013 Apr;36(4):998-1005. doi: 10.2337/dc12-0304. Epub 2012 Dec 13. Diabetes Care. 2013. PMID: 23238663 Free PMC article. Clinical Trial.
-
ACTB Methylation in Blood as a Potential Marker for the Pre-clinical Detection of Stroke: A Prospective Nested Case-Control Study.Front Neurosci. 2021 May 14;15:644943. doi: 10.3389/fnins.2021.644943. eCollection 2021. Front Neurosci. 2021. PMID: 34054407 Free PMC article.
-
Insulin, insulin resistance, and platelet signaling in diabetes.Diabetes Care. 2009 Apr;32(4):528-30. doi: 10.2337/dc08-1942. Diabetes Care. 2009. PMID: 19336637 Free PMC article. No abstract available.
-
Cytonemes Versus Neutrophil Extracellular Traps in the Fight of Neutrophils with Microbes.Int J Mol Sci. 2020 Jan 16;21(2):586. doi: 10.3390/ijms21020586. Int J Mol Sci. 2020. PMID: 31963289 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

