Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression
- PMID: 17502663
- PMCID: PMC2118605
- DOI: 10.1084/jem.20062129
Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression
Abstract
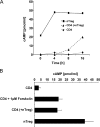
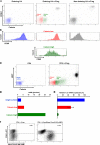
Naturally occurring regulatory T cells (T reg cells) are a thymus-derived subset of T cells, which are crucial for the maintenance of peripheral tolerance by controlling potentially autoreactive T cells. However, the underlying molecular mechanisms of this strictly cell contact-dependent process are still elusive. Here we show that naturally occurring T reg cells harbor high levels of cyclic adenosine monophosphate (cAMP). This second messenger is known to be a potent inhibitor of proliferation and interleukin 2 synthesis in T cells. Upon coactivation with naturally occurring T reg cells the cAMP content of responder T cells is also strongly increased. Furthermore, we demonstrate that naturally occurring T reg cells and conventional T cells communicate via cell contact-dependent gap junction formation. The suppressive activity of naturally occurring T reg cells is abolished by a cAMP antagonist as well as by a gap junction inhibitor, which blocks the cell contact-dependent transfer of cAMP to responder T cells. Accordingly, our results suggest that cAMP is crucial for naturally occurring T reg cell-mediated suppression and traverses membranes via gap junctions. Hence, naturally occurring T reg cells unexpectedly may control the immune regulatory network by a well-known mechanism based on the intercellular transport of cAMP via gap junctions.
Figures


References
-
- Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. - PubMed
-
- Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

