Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program
- PMID: 17502666
- PMCID: PMC2118610
- DOI: 10.1084/jem.20062545
Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program
Erratum in
- J Exp Med. 2007 Oct;204(10):2489
Abstract
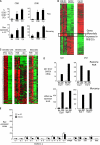
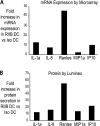
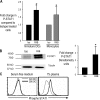
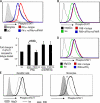
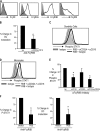
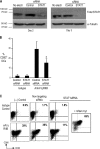
The ability of dendritic cells (DCs) to activate immunity is linked to their maturation status. In prior studies, we have shown that selective antibody-mediated blockade of inhibitory FcgammaRIIB receptor on human DCs in the presence of activating immunoglobulin (Ig) ligands leads to DC maturation and enhanced immunity to antibody-coated tumor cells. We show that Fcgamma receptor (FcgammaR)-mediated activation of human monocytes and monocyte-derived DCs is associated with a distinct gene expression pattern, including several inflammation-associated chemokines, as well as type 1 interferon (IFN) response genes, including the activation of signal transducer and activator of transcription 1 (STAT1). FcgammaR-mediated STAT1 activation is rapid and requires activating FcgammaRs. However, this IFN response is observed without a detectable increase in the expression of type I IFNs themselves or the need to add exogenous IFNs. Induction of IFN response genes plays an important role in FcgammaR-mediated effects on DCs, as suppression of STAT1 by RNA interference inhibited FcgammaR-mediated DC maturation. These data suggest that the balance of activating/inhibitory FcgammaRs may regulate IFN signaling in myeloid cells. Manipulation of FcgammaR balance on DCs and monocytes may provide a novel approach to regulating IFN-mediated pathways in autoimmunity and human cancer.
Figures
References
-
- Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. - PubMed
-
- Dhodapkar, K.M., J.L. Kaufman, M. Ehlers, D.K. Banerjee, E. Bonvini, S. Koenig, R.M. Steinman, J.V. Ravetch, and M.V. Dhodapkar. 2005. Selective blockade of inhibitory Fc gamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody- coated tumor cells. Proc. Natl. Acad. Sci. USA. 102:2910–2915. - PMC - PubMed
-
- Pestka, S., C.D. Krause, and M.R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8–32. - PubMed
-
- Platanias, L.C. 2005. Mechanisms of type-I- and type-II-interferon- mediated signalling. Nat. Rev. Immunol. 5:375–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

