The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins
- PMID: 17502670
- PMCID: PMC7122727
- DOI: 10.1007/978-1-59745-393-6_4
The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins
Abstract
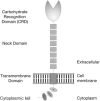
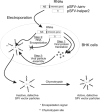
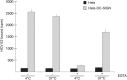
DC-SIGN and L-SIGN are C-type lectins that recognize carbohydrate structures present on viral glycoproteins and function as attachment factors for several enveloped viruses. DC-SIGN and L-SIGN enhance viral entry and facilitate infection of cells that express the cognate entry receptor (cis-infection). They are also able to capture viruses and transfer viral infections to other target cells (trans-infection). In this chapter, we will give an overview of protocols used to produce soluble viral glycoproteins at high levels and to study the molecular basis of viruses/DC-SIGN and L-SIGN binding and internalization. We will also describe techniques to investigate the molecular mechanisms by which DC-SIGN or L-SIGN spread viral infections.
Figures

Similar articles
-
L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14067-72. doi: 10.1073/pnas.0405695101. Epub 2004 Sep 15. Proc Natl Acad Sci U S A. 2004. PMID: 15371595 Free PMC article.
-
N-Glycans on the Rift Valley Fever Virus Envelope Glycoproteins Gn and Gc Redundantly Support Viral Infection via DC-SIGN.Viruses. 2016 May 23;8(5):149. doi: 10.3390/v8050149. Viruses. 2016. PMID: 27223297 Free PMC article.
-
DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans.J Virol. 2016 Aug 12;90(17):7848-63. doi: 10.1128/JVI.00537-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334579 Free PMC article.
-
The multiple facets of HIV attachment to dendritic cell lectins.Cell Microbiol. 2010 Nov;12(11):1553-61. doi: 10.1111/j.1462-5822.2010.01519.x. Epub 2010 Sep 20. Cell Microbiol. 2010. PMID: 20854332 Free PMC article. Review.
-
DC-SIGN, DC-SIGNR and LSECtin: C-type lectins for infection.Int Rev Immunol. 2014 Jan;33(1):54-66. doi: 10.3109/08830185.2013.834897. Epub 2013 Oct 24. Int Rev Immunol. 2014. PMID: 24156700 Review.
Cited by
-
Identification of a porcine DC-SIGN-related C-type lectin, porcine CLEC4G (LSECtin), and its order of intron removal during splicing: comparative genomic analyses of the cluster of genes CD23/CLEC4G/DC-SIGN among mammalian species.Dev Comp Immunol. 2009 Jun;33(6):747-60. doi: 10.1016/j.dci.2008.12.007. Epub 2009 Jan 21. Dev Comp Immunol. 2009. PMID: 19166875 Free PMC article.
-
Recent Advances in PRRS Virus Receptors and the Targeting of Receptor-Ligand for Control.Vaccines (Basel). 2021 Apr 7;9(4):354. doi: 10.3390/vaccines9040354. Vaccines (Basel). 2021. PMID: 33916997 Free PMC article. Review.
-
Cryo-EM Structures of Eastern Equine Encephalitis Virus Reveal Mechanisms of Virus Disassembly and Antibody Neutralization.Cell Rep. 2018 Dec 11;25(11):3136-3147.e5. doi: 10.1016/j.celrep.2018.11.067. Cell Rep. 2018. PMID: 30540945 Free PMC article.
-
Myeloid C-Type Lectin Receptors in Viral Recognition and Antiviral Immunity.Viruses. 2017 Mar 22;9(3):59. doi: 10.3390/v9030059. Viruses. 2017. PMID: 28327518 Free PMC article. Review.
-
The Immunomodulatory CEA Cell Adhesion Molecule 6 (CEACAM6/CD66c) Is a Protein Receptor for the Influenza a Virus.Viruses. 2021 Apr 21;13(5):726. doi: 10.3390/v13050726. Viruses. 2021. PMID: 33919410 Free PMC article.
References
-
- Soilleux E. J., Barten R., Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19pl3. J. Immunol. 2000;165:2937–2942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

