Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa
- PMID: 17502726
- PMCID: PMC2486349
- DOI: 10.1097/QAD.0b013e3281c615da
Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa
Abstract
Objectives: To analyse the association between the presence of resistance mutations and treatment outcomes. The impact of HIV-1 drug resistance mutations in African adults on HAART has so far never been reported.
Methods: In 2004 in Abidjan, Côte d'Ivoire, 106 adults on HAART had plasma viral load measurements. Patients with detectable viral loads had resistance genotypic tests. Patients were followed until 2006. Main outcomes were serious morbidity and immunological failure (CD4 cell count < 200 cells/microl).
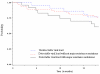
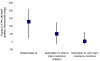
Results: At study entry, the median previous time on HAART was 37 months and the median CD4 cell count was 266 cells/microl; 58% of patients had undetectable viral loads, 20% had detectable viral loads with no major resistance mutations, and 22% had detectable viral loads with one or more major mutations. The median change in CD4 cell count between study entry and study termination was +129 cells/microl in patients with undetectable viral loads, +51 cells/microl in those with detectable viral loads with no mutations and +3 cells/microl in those with detectable viral loads with resistance mutations. Compared with patients with undetectable viral loads, those with detectable viral loads with resistance mutations had adjusted hazard ratios of immunological failure of 4.32 (95%CI 1.38-13.57, P = 0.01). One patient died. The 18-month probability of remaining free of morbidity was 0.79 in patients with undetectable viral loads and 0.69 in those with resistance mutations (P = 0.19).
Conclusion: In this setting with restricted access to second-line HAART, patients with major resistance mutations had higher rates of immunological failure, but most maintained stable CD4 cell counts and stayed alive for at least 20 months.
Figures
Comment in
-
When to switch for antiretroviral treatment failure in resource-limited settings?AIDS. 2007 May 31;21(9):1205-6. doi: 10.1097/QAD.0b013e3281c617e8. AIDS. 2007. PMID: 17502731 No abstract available.
References
-
- UNAIDS. Report on the global AIDS epidemic; Geneva. 2006. Available at: http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
-
- Nkengasong JAN, Adje-Toure C, Weidle PJ. HIV antiretroviral drug resistance in Africa. AIDS Rev. 2004;6:4–12. - PubMed
-
- Toni T, Masquelier B, Bonard D, et al. Primary HIV-1 drug resistance in Abidjan (Cote d’Ivoire): a genotypic and phenotypic study. AIDS. 2002;16:488–491. - PubMed
-
- Vergne L, Kane CT, Laurent C, et al. Low rate of genotypic HIV-1 drug-resistant strains in the Senegalese government initiative of access to antiretroviral therapy. AIDS. 2003;17 (Suppl 3):S31–38. - PubMed
-
- Bellocchi MC, Forbici F, Palombi L, et al. Subtype analysis and mutations to antiviral drugs in HIV-1-infected patients from Mozambique before initiation of antiretroviral therapy: results from the DREAM programme. J Med Virol. 2005;76:452–458. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials