Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years
- PMID: 17502917
- PMCID: PMC1855989
- DOI: 10.1371/journal.pone.0000435
Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years
Abstract
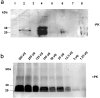
The persistence of infectious biomolecules in soil constitutes a substantial challenge. This holds particularly true with respect to prions, the causative agents of transmissible spongiform encephalopathies (TSEs) such as scrapie, bovine spongiform encephalopathy (BSE), or chronic wasting disease (CWD). Various studies have indicated that prions are able to persist in soil for years without losing their pathogenic activity. Dissemination of prions into the environment can occur from several sources, e.g., infectious placenta or amniotic fluid of sheep. Furthermore, environmental contamination by saliva, excrements or non-sterilized agricultural organic fertilizer is conceivable. Natural transmission of scrapie in the field seems to occur via the alimentary tract in the majority of cases, and scrapie-free sheep flocks can become infected on pastures where outbreaks of scrapie had been observed before. These findings point to a sustained contagion in the environment, and notably the soil. By using outdoor lysimeters, we simulated a contamination of standard soil with hamster-adapted 263K scrapie prions, and analyzed the presence and biological activity of the soil-associated PrP(Sc) and infectivity by Western blotting and hamster bioassay, respectively. Our results showed that 263K scrapie agent can persist in soil at least over 29 months. Strikingly, not only the contaminated soil itself retained high levels of infectivity, as evidenced by oral administration to Syrian hamsters, but also feeding of aqueous soil extracts was able to induce disease in the reporter animals. We could also demonstrate that PrP(Sc) in soil, extracted after 21 months, provides a catalytically active seed in the protein misfolding cyclic amplification (PMCA) reaction. PMCA opens therefore a perspective for considerably improving the detectability of prions in soil samples from the field.
Conflict of interest statement
Figures


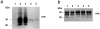
References
-
- Miller MW, Williams ES, Sigurdson CJ. Prion disease: horizontal prion transmission in mule deer. Other animal prion diseases. Nature. 2003;425:35–36. - PubMed
-
- Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech. 2002;21:305–316. - PubMed
-
- Williams ES. Chronic wasting disease. Vet Pathol. 2005;42:530–549. - PubMed
-
- Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech. 1996;15:827–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

