On the dynamics of the spontaneous activity in neuronal networks
- PMID: 17502919
- PMCID: PMC1857824
- DOI: 10.1371/journal.pone.0000439
On the dynamics of the spontaneous activity in neuronal networks
Abstract
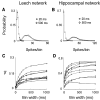
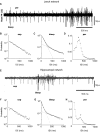
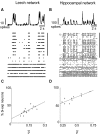
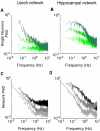
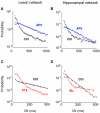
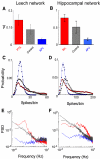
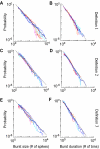
Most neuronal networks, even in the absence of external stimuli, produce spontaneous bursts of spikes separated by periods of reduced activity. The origin and functional role of these neuronal events are still unclear. The present work shows that the spontaneous activity of two very different networks, intact leech ganglia and dissociated cultures of rat hippocampal neurons, share several features. Indeed, in both networks: i) the inter-spike intervals distribution of the spontaneous firing of single neurons is either regular or periodic or bursting, with the fraction of bursting neurons depending on the network activity; ii) bursts of spontaneous spikes have the same broad distributions of size and duration; iii) the degree of correlated activity increases with the bin width, and the power spectrum of the network firing rate has a 1/f behavior at low frequencies, indicating the existence of long-range temporal correlations; iv) the activity of excitatory synaptic pathways mediated by NMDA receptors is necessary for the onset of the long-range correlations and for the presence of large bursts; v) blockage of inhibitory synaptic pathways mediated by GABA(A) receptors causes instead an increase in the correlation among neurons and leads to a burst distribution composed only of very small and very large bursts. These results suggest that the spontaneous electrical activity in neuronal networks with different architectures and functions can have very similar properties and common dynamics.
Conflict of interest statement
Figures
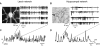

Similar articles
-
Network burst activity in hippocampal neuronal cultures: the role of synaptic and intrinsic currents.J Neurophysiol. 2016 Jun 1;115(6):3073-89. doi: 10.1152/jn.00995.2015. Epub 2016 Mar 16. J Neurophysiol. 2016. PMID: 26984425 Free PMC article.
-
Homeostatic presynaptic suppression of neuronal network bursts.J Neurophysiol. 2009 Apr;101(4):2077-88. doi: 10.1152/jn.91085.2008. Epub 2009 Feb 4. J Neurophysiol. 2009. PMID: 19193770
-
Recurrently connected and localized neuronal communities initiate coordinated spontaneous activity in neuronal networks.PLoS Comput Biol. 2017 Jul 27;13(7):e1005672. doi: 10.1371/journal.pcbi.1005672. eCollection 2017 Jul. PLoS Comput Biol. 2017. PMID: 28749937 Free PMC article.
-
Compensatory physiological responses to chronic blockade of amino acid receptors during early development in spontaneously active organotypic cerebral cortex explants cultured in vitro.Prog Brain Res. 2005;147:231-48. doi: 10.1016/S0079-6123(04)47018-6. Prog Brain Res. 2005. PMID: 15581710 Review.
-
Spontaneous signalling in small central neurons: mechanisms and roles of spike-amplitude and spike-interval fluctuations.Int J Neural Syst. 1996 Sep;7(4):369-76. doi: 10.1142/s0129065796000336. Int J Neural Syst. 1996. PMID: 8968826 Review.
Cited by
-
Temporal correlations in neuronal avalanche occurrence.Sci Rep. 2016 Apr 20;6:24690. doi: 10.1038/srep24690. Sci Rep. 2016. PMID: 27094323 Free PMC article.
-
Advances in the application of technology to epilepsy: the CIMIT/NIO Epilepsy Innovation Summit.Epilepsy Behav. 2009 Sep;16(1):3-46. doi: 10.1016/j.yebeh.2009.06.028. Epilepsy Behav. 2009. PMID: 19780225 Free PMC article. Review.
-
A coarse-graining framework for spiking neuronal networks: from strongly-coupled conductance-based integrate-and-fire neurons to augmented systems of ODEs.J Comput Neurosci. 2019 Apr;46(2):211-232. doi: 10.1007/s10827-019-00712-w. Epub 2019 Feb 16. J Comput Neurosci. 2019. PMID: 30788694
-
Landau-Ginzburg theory of cortex dynamics: Scale-free avalanches emerge at the edge of synchronization.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1356-E1365. doi: 10.1073/pnas.1712989115. Epub 2018 Jan 29. Proc Natl Acad Sci U S A. 2018. PMID: 29378970 Free PMC article.
-
Self-organized criticality in developing neuronal networks.PLoS Comput Biol. 2010 Dec 2;6(12):e1001013. doi: 10.1371/journal.pcbi.1001013. PLoS Comput Biol. 2010. PMID: 21152008 Free PMC article.
References
-
- Raichle M. The brain dark energy. Science. 2006;314:1249–1250. - PubMed
-
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. - PubMed
-
- Calabrese RL, Nadim F, Olsen OH. Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination and modulation of rhythmic motor patterns. J Neurobiol. 1995;27:390–402. - PubMed
-
- Bianchi AL, Denavit Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. J Am Phys Soc. 1995;75:1–4. - PubMed
-
- Kristan WB, Jr, Calabrese RI, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

