Measurement of cerebral perfusion territories using arterial spin labelling
- PMID: 17503440
- PMCID: PMC4756389
- DOI: 10.1002/nbm.1177
Measurement of cerebral perfusion territories using arterial spin labelling
Abstract
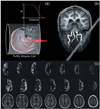
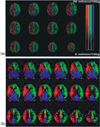
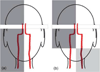
The ability to assess the perfusion territories of major cerebral arteries can be a valuable asset to the diagnosis of a number of cerebrovascular diseases. Recently, several arterial spin labeling (ASL) techniques have been proposed for determining the cerebral perfusion territories of individual arteries by three different approaches: (1) using a dedicated labeling radio frequency (RF) coil; (2) applying selective inversion of spatially confined areas; (3) employing multidimensional RF pulses. Methods that use a separate labeling RF coil have high signal-to-noise ratio (SNR), low RF power deposition, and unrestricted three-dimensional coverage, but are mostly limited to separation of the left and right circulation, and do require extra hardware, which may limit their implementation in clinical systems. Alternatively, methods that utilize selective inversion have higher flexibility of implementation and higher arterial selectivity, but suffer from imaging artifacts resulting from interference between the labeling slab and the volume of interest. The goal of this review is to provide the reader with a critical survey of the different ASL approaches proposed to date for determining cerebral perfusion territories, by discussing the relative advantages and disadvantages of each technique, so as to serve as a guide for future refinement of this promising methodology.
Copyright 2007 John Wiley & Sons, Ltd.
Figures





References
-
- Hoeffner EG. Cerebral perfusion imaging. J Neuroophthalmol. 2005;25(4):313–320. - PubMed
-
- Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36(9):e83–e99. - PubMed
-
- Cha S. Perfusion MR imaging: basic principles and clinical applications. Magn Reson Imaging Clin N Am. 2003;11(3):403–413. - PubMed
-
- Lad SP, Guzman R, Kelly ME, Li G, Lim M, Lovbald K, Steinberg GK. Cerebral perfusion imaging in vasospasm. Neurosurg Focus. 2006;21(3):E7. - PubMed
-
- van Laar PJ, Hendrikse J, Golay X, Lu H, van Osch MJ, van der Grond J. In vivo flow territory mapping of major brain feeding arteries. Neuroimage. 2006;29(1):136–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

