Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria
- PMID: 17504445
- PMCID: PMC2517299
- DOI: 10.1111/j.1365-2613.2007.00530.x
Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria
Abstract
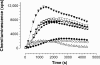
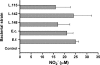
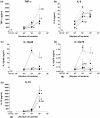
Chronic active colitis (including inflammatory bowel disease - IBD) is maintained by a variety of pro-inflammatory mediators. Certain intestinal bacterial strains may induce colitis, whereas some strains (e.g. Lactobacillus spp.) show a protective effect in colitis owing to their anti-inflammatory activity. In this study, we have examined the production of selected inflammatory cytokines, reactive oxygen species (ROS), nitric oxide (NO) and the expression of haeme oxygenase-1 (HO-1) by murine peritoneal macrophages stimulated in vitro by the intestinal bacterial strains, isolated from mice with colitis. Lactobacillus strains (Lactobacillus reuteri, L. johnsonii, L. animalis/murinus) and two potentially pathogenic bacteria (Escherichia coli and Enterococcus faecalis) induced the production of substantial amounts of cytokines with a strain specific profile. Despite some interstrain differences, all lactobacilli induced production of anti-inflammatory cytokines (IL-10(high), IL-6(low), IL-12p70(low)). Conversely, E. faecalis and E. coli induced the production of proinflammatory cytokines (TNF-alpha, IL-12p70), the cytokines essential for chronic IBD. Macrophages released comparably substantial amounts of ROS in response to all Lactobacillus strains tested, while E. coli and E. faecalis ability to induce generation of ROS was negligible. In contrast to ROS, the production of NO/NO(2) (-) by macrophages activated with all bacterial strains tested was similar. Moreover, for the first time, it has been shown that intestinal bacteria differed in their ability to induce expression of HO-1, a stress-inducible enzyme with antioxidant and anti-inflammatory properties. The beneficial immunoregulatory properties of candidate probiotic bacteria for the treatment of IBD are discussed.
Figures

References
-
- Albrecht D, Jungi TW. Luminol-enhanced chemiluminescence induced peripheral blood-derived human phagocytes: obligatory requirement of myeloperoxidase endocytosis by monocytes. J. Leukoc. Biol. 1993;54:300–306. - PubMed
-
- Baumgart DC, Dignass AU. Intestinal barrier function. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:685–694. - PubMed
-
- Becker C, Dornhoff H, Neufert C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 2006;177:2760–2764. - PubMed
-
- Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 2005;100:1539–1546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

