Antisense oligonucleotide inhibition of heat shock protein (HSP) 47 improves bleomycin-induced pulmonary fibrosis in rats
- PMID: 17504519
- PMCID: PMC1876458
- DOI: 10.1186/1465-9921-8-37
Antisense oligonucleotide inhibition of heat shock protein (HSP) 47 improves bleomycin-induced pulmonary fibrosis in rats
Abstract
Background: The most common pathologic form of pulmonary fibrosis arises from excessive deposition of extracellular matrix proteins such as collagen. The 47 kDa heat shock protein 47 (HSP47) is a collagen-specific molecular chaperone that has been shown to play a major role during the processing and/or secretion of procollagen.
Objectives: To determine whether inhibition of HSP47 could have beneficial effects in mitigating bleomycin-induced pulmonary fibrosis in rats.
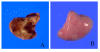
Methods: All experiments were performed with 250-300 g male Wistar rats. Animals were randomly divided into five experimental groups that were administered: 1) saline alone, 2) bleomycin alone, 3) antisense HSP47 oligonucleotides alone, 4) bleomycin + antisense HSP47 oligonucleotides, and 5) bleomycin + sense control oligonucleotides. We investigated lung histopathology and performed immunoblot and immunohistochemistry analyses.
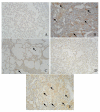
Results: In rats treated with HSP47 antisense oligonucleotides, pulmonary fibrosis was significantly reduced. In addition, treatment with HSP47 antisense oligonucleotides significantly improved bleomycin-induced morphological changes. Treatment with HSP47 antisense oligonucleotides alone did not produce any significant changes to lung morphology. Immunoblot analyses of lung homogenates confirmed the inhibition of HSP47 protein by antisense oligonucleotides. The bleo + sense group, however, did not exhibit any improvement in lung pathology compared to bleomycin alone groups, and also had no effect on HSP47 expression.
Conclusion: These findings suggest that HSP47 antisense oligonucleotide inhibition of HSP47 improves bleomycin-induced pulmonary fibrosis pathology in rats.
Figures





Similar articles
-
An antisense oligonucleotide to HSP47 inhibits paraquat-induced pulmonary fibrosis in rats.Toxicology. 2007 Jul 17;236(3):199-207. doi: 10.1016/j.tox.2007.04.013. Epub 2007 May 1. Toxicology. 2007. PMID: 17543438
-
Heat shock protein 47 (HSP47) antisense oligonucleotides reduce cardiac remodeling and improve cardiac function in a rat model of myocardial infarction.Thorac Cardiovasc Surg. 2011 Oct;59(7):386-92. doi: 10.1055/s-0030-1250658. Epub 2011 Mar 16. Thorac Cardiovasc Surg. 2011. PMID: 21412710
-
Introduction of antisense oligonucleotides to heat shock protein 47 prevents pulmonary fibrosis in lipopolysaccharide-induced pneumopathy of the rat.Eur J Pharmacol. 2007 Jun 14;564(1-3):174-80. doi: 10.1016/j.ejphar.2007.02.057. Epub 2007 Mar 12. Eur J Pharmacol. 2007. Retraction in: Eur J Pharmacol. 2016 Dec 5;792:80. doi: 10.1016/j.ejphar.2016.11.022. PMID: 17400207 Retracted.
-
Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis.Biochem Soc Trans. 2002 Apr;30(2):211-6. Biochem Soc Trans. 2002. PMID: 12023853 Review.
-
HSP47: a potential target for fibrotic diseases and implications for therapy.Expert Opin Ther Targets. 2021 Jan;25(1):49-62. doi: 10.1080/14728222.2021.1861249. Epub 2021 Jan 4. Expert Opin Ther Targets. 2021. PMID: 33287600 Review.
Cited by
-
Heat shock protein 47 promotes tumor survival and therapy resistance by modulating AKT signaling via PHLPP1 in colorectal cancer.Cancer Biol Med. 2020 May 15;17(2):343-356. doi: 10.20892/j.issn.2095-3941.2019.0261. Cancer Biol Med. 2020. PMID: 32587773 Free PMC article.
-
Fibrocytes in the fibrotic lung: altered phenotype detected by flow cytometry.Front Pharmacol. 2014 Jun 16;5:141. doi: 10.3389/fphar.2014.00141. eCollection 2014. Front Pharmacol. 2014. PMID: 24999331 Free PMC article.
-
Heat shock protein 47 regulated by miR-29a to enhance glioma tumor growth and invasion.J Neurooncol. 2014 May;118(1):39-47. doi: 10.1007/s11060-014-1412-7. Epub 2014 Mar 5. J Neurooncol. 2014. PMID: 24595468
-
Drug like HSP27 cross linkers with chromenone structure ameliorates pulmonary fibrosis.Front Pharmacol. 2023 Jul 4;14:1203033. doi: 10.3389/fphar.2023.1203033. eCollection 2023. Front Pharmacol. 2023. PMID: 37469871 Free PMC article.
-
Serum heat shock protein 47 levels are elevated in acute exacerbation of idiopathic pulmonary fibrosis.Cell Stress Chaperones. 2013 Sep;18(5):581-90. doi: 10.1007/s12192-013-0411-5. Epub 2013 Feb 22. Cell Stress Chaperones. 2013. PMID: 23435730 Free PMC article.
References
-
- Specks U, Nerlich A, Colby TV, Wiest I, Timpl R. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995;151:1956–1964. - PubMed
-
- Xu YD, Hua J, Mui A, O'Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:527–539. - PubMed
-
- Natsume T, Koide T, Yokota S, Hirayshi K, Nagata K. Interactions between collagen-binding stress protein HSP47 and collagen. Analysis of kinetic parameters by surface plasmon resonance biosensor. J Biol Chem. 1994;269:31224–31228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

