Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65
- PMID: 17504753
- PMCID: PMC2747659
- DOI: 10.1074/jbc.M701432200
Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65
Abstract
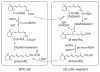
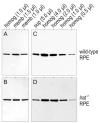
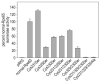
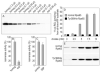
Absorption of a photon by a vertebrate opsin pigment induces 11-cis to all-trans isomerization of its retinaldehyde chromophore. Restoration of light sensitivity to the bleached opsin requires chemical re-isomerization of the chromophore via an enzyme pathway called the visual cycle. The retinoid isomerase in this pathway is Rpe65, a membrane-associated protein in the retinal pigment epithelium (RPE) with no predicted membrane-spanning segments. It has been suggested that Rpe65 is S-palmitoylated by lecithin:retinol acyl transferase (LRAT) on Cys(231), Cys(329), and Cys(330), and that this palmitoylation is required for isomerase activity and the association of Rpe65 with membranes. Here we show that the affinity of Rpe65 for membranes is similar in wild-type and lrat(-/-) mice. The isomerase activity of Rpe65 is also similar in both strains when all-trans-retinyl palmitate is used as substrate. With all-trans-retinol substrate, isomerase activity is present in wild-type but undetectable in RPE homogenates from lrat(-/-) mice. Substitution of Cys(231), Cys(329), and Cys(330) with Ser or Ala did not affect the affinity of Rpe65 for membranes. Further, these Cys residues are not palmitoylated in Rpe65 by mass spectrometric analysis. Global inhibition of protein palmitoylation by 2-bromopalmitate did not affect the solubility or isomerase activity of Rpe65. Finally, we show that soluble and membrane-associated Rpe65 possesses similar isomerase specific activities. These results indicate that LRAT is not required for isomerase activity beyond synthesis of retinyl-ester substrate, and that the association of Rpe65 with membranes is neither dependent upon LRAT nor the result of S-palmitoylation. The affinity of Rpe65 for membranes is probably an intrinsic feature of this protein.
Figures





Similar articles
-
Rpe65 isomerase associates with membranes through an electrostatic interaction with acidic phospholipid headgroups.J Biol Chem. 2010 Jan 8;285(2):988-99. doi: 10.1074/jbc.M109.025643. Epub 2009 Nov 5. J Biol Chem. 2010. PMID: 19892706 Free PMC article.
-
Analysis of the retinoid isomerase activities in the retinal pigment epithelium and retina.Methods Mol Biol. 2010;652:329-39. doi: 10.1007/978-1-60327-325-1_19. Methods Mol Biol. 2010. PMID: 20552438 Free PMC article.
-
Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells.J Biol Chem. 2008 Jul 11;283(28):19730-8. doi: 10.1074/jbc.M801288200. Epub 2008 May 12. J Biol Chem. 2008. PMID: 18474598 Free PMC article.
-
Lecithin:Retinol Acyltransferase: A Key Enzyme Involved in the Retinoid (visual) Cycle.Biochemistry. 2016 Jun 7;55(22):3082-91. doi: 10.1021/acs.biochem.6b00319. Epub 2016 May 23. Biochemistry. 2016. PMID: 27183166 Free PMC article. Review.
-
RPE65 Palmitoylation: A Tale of Lipid Posttranslational Modification.Adv Exp Med Biol. 2019;1185:537-541. doi: 10.1007/978-3-030-27378-1_88. Adv Exp Med Biol. 2019. PMID: 31884667 Free PMC article. Review.
Cited by
-
Mechanisms of retinal photoreceptor loss in spontaneously hypertensive rats.Exp Eye Res. 2024 Oct;247:110065. doi: 10.1016/j.exer.2024.110065. Epub 2024 Aug 31. Exp Eye Res. 2024. PMID: 39222765
-
Rpe65 isomerase associates with membranes through an electrostatic interaction with acidic phospholipid headgroups.J Biol Chem. 2010 Jan 8;285(2):988-99. doi: 10.1074/jbc.M109.025643. Epub 2009 Nov 5. J Biol Chem. 2010. PMID: 19892706 Free PMC article.
-
RPE65: role in the visual cycle, human retinal disease, and gene therapy.Ophthalmic Genet. 2009 Jun;30(2):57-62. doi: 10.1080/13816810802626399. Ophthalmic Genet. 2009. PMID: 19373675 Free PMC article. Review.
-
Predicting the pathogenicity of RPE65 mutations.Hum Mutat. 2009 Aug;30(8):1183-8. doi: 10.1002/humu.21033. Hum Mutat. 2009. PMID: 19431183 Free PMC article.
-
Rescue of enzymatic function for disease-associated RPE65 proteins containing various missense mutations in non-active sites.J Biol Chem. 2014 Jul 4;289(27):18943-56. doi: 10.1074/jbc.M114.552117. Epub 2014 May 21. J Biol Chem. 2014. PMID: 24849605 Free PMC article.
References
-
- Saari JC, Bredberg DL. J. Biol. Chem. 1989;264:8636–8640. - PubMed
-
- Gollapalli DR, Rando RR. Biochemistry. 2003;42:5809–5818. - PubMed
-
- Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr., Redmond TM, Ma JX. Biochemistry. 2003;42:2229–2238. - PubMed
-
- Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. J. Biol. Chem. 2004;279:635–643. - PubMed
-
- Rando RR. Biochemistry. 1991;30:595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

