The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development
- PMID: 17504941
- PMCID: PMC1865495
- DOI: 10.1101/gad.1543607
The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development
Abstract
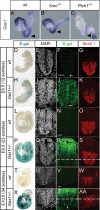
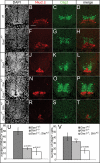
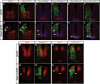
Hedgehog (Hh) signaling is critical for patterning and growth during mammalian embryogenesis. Transcriptional profiling identified Growth-arrest-specific 1 (Gas1) as a general negative target of Shh signaling. Data presented here define Gas1 as a novel positive component of the Shh signaling cascade. Removal of Gas1 results in a Shh dose-dependent loss of cell identities in the ventral neural tube and facial and skeletal defects, also consistent with reduced Shh signaling. In contrast, ectopic Gas1 expression results in Shh-dependent cell-autonomous promotion of ventral cell identities. These properties mirror those of Cdo, an unrelated, cell surface Shh-binding protein. We show that Gas1 and Cdo cooperate to promote Shh signaling during neural tube patterning, craniofacial, and vertebral development. Overall, these data support a new paradigm in Shh signaling whereby positively acting ligand-binding components, which are initially expressed in responding tissues to promote signaling, are then down-regulated by active Hh signaling, thereby modulating responses to ligand input.
Figures




References
-
- Ahn S., Joyner A.L., Joyner A.L. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
-
- Ashe H.L., Briscoe J., Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. - PubMed
-
- Bottcher R.T., Niehrs C., Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005;26:63–77. - PubMed
-
- Briscoe J., Ericson J., Ericson J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001;11:43–49. - PubMed
-
- Briscoe J., Pierani A., Jessell T.M., Ericson J., Pierani A., Jessell T.M., Ericson J., Jessell T.M., Ericson J., Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
