Environmental regulation operating at the promoter clearance step of bacterial transcription
- PMID: 17504942
- PMCID: PMC1865496
- DOI: 10.1101/gad.1520507
Environmental regulation operating at the promoter clearance step of bacterial transcription
Abstract
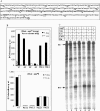
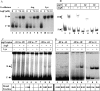
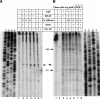
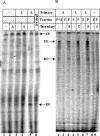
In vivo transcription of the Escherichia coli argO gene, which encodes an arginine (Arg) exporter, requires the LysR-family regulator protein ArgP (previously called IciA) and is induced in the presence of Arg or its naturally occurring antimetabolite analog canavanine. Lysine (Lys) addition, on the other hand, phenocopies an argP mutation to result in the shutoff of argO expression. We now report that the ArgP dimer by itself is able to bind the argO promoter-operator region to form a binary complex, but that the formation of a ternary complex with RNA polymerase is greatly stimulated only in presence of a coeffector. Both Arg and Lys were proficient as coeffectors for ArgP-mediated recruitment of RNA polymerase to, and open complex formation at, the argO promoter, although only Arg (but not Lys) was competent to activate transcription. The two coeffectors competed for binding to ArgP, and the ternary complex that had been assembled on the argO template in the presence of Lys could be chased into a transcriptionally active state upon Arg addition. Our results support a novel mechanism of argO regulation in which Lys-bound ArgP reversibly restrains RNA polymerase at the promoter, at a step (following open complex formation) that precedes, and is common to, both abortive and productive transcription. This represents, therefore, the first example of an environmental signal regulating the final step of promoter clearance by RNA polymerase in bacterial transcription. We propose that, in E. coli cells, the ternary complex remains assembled and poised at the argO promoter at all times to respond, positively or negatively, to instantaneous changes in the ratio of intracellular Arg to Lys concentrations.
Figures



Similar articles
-
Distinct Paths for Basic Amino Acid Export in Escherichia coli: YbjE (LysO) Mediates Export of L-Lysine.J Bacteriol. 2015 Jun 15;197(12):2036-47. doi: 10.1128/JB.02505-14. Epub 2015 Apr 6. J Bacteriol. 2015. PMID: 25845847 Free PMC article.
-
Transcriptional cross-regulation between Gram-negative and gram-positive bacteria, demonstrated using ArgP-argO of Escherichia coli and LysG-lysE of Corynebacterium glutamicum.J Bacteriol. 2012 Oct;194(20):5657-66. doi: 10.1128/JB.00947-12. Epub 2012 Aug 17. J Bacteriol. 2012. PMID: 22904281 Free PMC article.
-
Differential protein-DNA contacts for activation and repression by ArgP, a LysR-type (LTTR) transcriptional regulator in Escherichia coli.Microbiol Res. 2018 Jan;206:141-158. doi: 10.1016/j.micres.2017.10.009. Epub 2017 Oct 23. Microbiol Res. 2018. PMID: 29146251
-
Evidence for an arginine exporter encoded by yggA (argO) that is regulated by the LysR-type transcriptional regulator ArgP in Escherichia coli.J Bacteriol. 2004 Jun;186(11):3539-46. doi: 10.1128/JB.186.11.3539-3546.2004. J Bacteriol. 2004. PMID: 15150242 Free PMC article.
-
Transcription regulation by repressosome and by RNA polymerase contact.Cold Spring Harb Symp Quant Biol. 1998;63:1-9. doi: 10.1101/sqb.1998.63.1. Cold Spring Harb Symp Quant Biol. 1998. PMID: 10384265 Review.
Cited by
-
Diversity, versatility and complexity of bacterial gene regulation mechanisms: opportunities and drawbacks for applications in synthetic biology.FEMS Microbiol Rev. 2019 May 1;43(3):304-339. doi: 10.1093/femsre/fuz001. FEMS Microbiol Rev. 2019. PMID: 30721976 Free PMC article. Review.
-
Transcriptome analysis of Vibrio parahaemolyticus in type III secretion system 1 inducing conditions.Front Cell Infect Microbiol. 2014 Jan 20;4:1. doi: 10.3389/fcimb.2014.00001. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24478989 Free PMC article.
-
Distinct Paths for Basic Amino Acid Export in Escherichia coli: YbjE (LysO) Mediates Export of L-Lysine.J Bacteriol. 2015 Jun 15;197(12):2036-47. doi: 10.1128/JB.02505-14. Epub 2015 Apr 6. J Bacteriol. 2015. PMID: 25845847 Free PMC article.
-
Transcriptional cross-regulation between Gram-negative and gram-positive bacteria, demonstrated using ArgP-argO of Escherichia coli and LysG-lysE of Corynebacterium glutamicum.J Bacteriol. 2012 Oct;194(20):5657-66. doi: 10.1128/JB.00947-12. Epub 2012 Aug 17. J Bacteriol. 2012. PMID: 22904281 Free PMC article.
-
The poly A polymerase Star-PAP controls 3'-end cleavage by promoting CPSF interaction and specificity toward the pre-mRNA.EMBO J. 2010 Dec 15;29(24):4132-45. doi: 10.1038/emboj.2010.287. Epub 2010 Nov 19. EMBO J. 2010. PMID: 21102410 Free PMC article.
References
-
- Aleshin V.V., Zakataeva N.P., Livshits V.A., Zakataeva N.P., Livshits V.A., Livshits V.A. A new family of amino-acid-efflux proteins. Trends Biochem. Sci. 1999;24:133–135. - PubMed
-
- Azam T.A., Ishihama A., Ishihama A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J. Biol. Chem. 1999;274:33105–33113. - PubMed
-
- Azam T.A., Iwata A., Nishimura A., Ueda S., Ishihama A., Iwata A., Nishimura A., Ueda S., Ishihama A., Nishimura A., Ueda S., Ishihama A., Ueda S., Ishihama A., Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleotid. J. Bacteriol. 1999;181:6361–6370. - PMC - PubMed
-
- Bellmann A., Vrljić M., Patek M., Sahm H., Krämer R., Eggeling L., Vrljić M., Patek M., Sahm H., Krämer R., Eggeling L., Patek M., Sahm H., Krämer R., Eggeling L., Sahm H., Krämer R., Eggeling L., Krämer R., Eggeling L., Eggeling L. Expression control and specificity of the basic amino acid exporter LysE of Corynebacterium glutamicum. Microbiol. 2001;147:1765–1774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
