Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor
- PMID: 17505061
- PMCID: PMC3839341
- DOI: 10.1210/me.2006-0467
Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor
Abstract
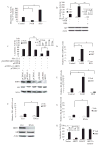
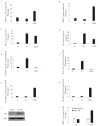
Androgen antagonists or androgen deprivation is a primary therapeutic modality for the treatment of prostate cancer. Invariably, however, the disease becomes progressive and unresponsive to androgen ablation therapy (hormone refractory). The molecular mechanisms by which the androgen antagonists inhibit prostate cancer proliferation are not fully defined. In this report, we demonstrate that sirtuin 1 (SIRT1), a nicotinamide adenosine dinucleotide-dependent histone deacetylase (HDAC) linked to the regulation of longevity, is required for androgen antagonist-mediated transcriptional repression and growth suppression. Androgen antagonist-bound androgen receptor (AR) recruits SIRT1 and nuclear receptor corepressor to AR-responsive promoters and deacetylates histone H3 locally at the prostate-specific antigen promoter. Furthermore, SIRT1 down-regulation by small interfering RNA or by pharmacological means increased the sensitivity of androgen-responsive genes to androgen stimulation, enhanced the sensitivity of prostate cancer cell proliferative responses to androgens, and decreased the sensitivity of prostate cancer cells to androgen antagonists. In this study, we demonstrate the ligand-dependent recruitment of a class III HDAC into a corepressor transcriptional complex and a necessary functional role for a class III HDAC as a transcriptional corepressor in AR antagonist-induced transcriptional repression. Collectively, these findings identify SIRT1 as a corepressor of AR and elucidate a new molecular pathway relevant to prostate cancer growth and approaches to therapy.
Figures
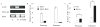


Similar articles
-
Prohibitin and the SWI/SNF ATPase subunit BRG1 are required for effective androgen antagonist-mediated transcriptional repression of androgen receptor-regulated genes.Carcinogenesis. 2008 Sep;29(9):1725-33. doi: 10.1093/carcin/bgn117. Epub 2008 May 16. Carcinogenesis. 2008. PMID: 18487222 Free PMC article.
-
Inhibition of MAPK-signaling pathway promotes the interaction of the corepressor SMRT with the human androgen receptor and mediates repression of prostate cancer cell growth in the presence of antiandrogens.J Mol Endocrinol. 2009 May;42(5):429-35. doi: 10.1677/JME-08-0084. Epub 2009 Feb 17. J Mol Endocrinol. 2009. PMID: 19223455
-
The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor.Mol Endocrinol. 2006 May;20(5):1048-60. doi: 10.1210/me.2005-0324. Epub 2005 Dec 22. Mol Endocrinol. 2006. PMID: 16373395
-
Androgen receptor: role and novel therapeutic prospects in prostate cancer.Expert Rev Anticancer Ther. 2008 Sep;8(9):1495-508. doi: 10.1586/14737140.8.9.1495. Expert Rev Anticancer Ther. 2008. PMID: 18759700 Review.
-
Androgen receptor in prostate cancer.Endocr Rev. 2004 Apr;25(2):276-308. doi: 10.1210/er.2002-0032. Endocr Rev. 2004. PMID: 15082523 Review.
Cited by
-
Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer.Med Oncol. 2012 Dec;29(5):3240-9. doi: 10.1007/s12032-012-0260-6. Epub 2012 Jun 4. Med Oncol. 2012. PMID: 22661383
-
Enhanced cytotoxicity from deoxyguanosine-enriched T-oligo in prostate cancer cells.Nucleic Acid Ther. 2013 Oct;23(5):311-21. doi: 10.1089/nat.2013.0420. Epub 2013 Aug 24. Nucleic Acid Ther. 2013. PMID: 23971906 Free PMC article.
-
KAT5 and KAT6B are in positive regulation on cell proliferation of prostate cancer through PI3K-AKT signaling.Int J Clin Exp Pathol. 2013 Nov 15;6(12):2864-71. eCollection 2013. Int J Clin Exp Pathol. 2013. PMID: 24294372 Free PMC article.
-
Prohibitin and the SWI/SNF ATPase subunit BRG1 are required for effective androgen antagonist-mediated transcriptional repression of androgen receptor-regulated genes.Carcinogenesis. 2008 Sep;29(9):1725-33. doi: 10.1093/carcin/bgn117. Epub 2008 May 16. Carcinogenesis. 2008. PMID: 18487222 Free PMC article.
-
Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation.Cancer Res. 2011 Feb 1;71(3):964-75. doi: 10.1158/0008-5472.CAN-10-3172. Epub 2010 Dec 28. Cancer Res. 2011. PMID: 21189328 Free PMC article.
References
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. - PubMed
-
- Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994;44(1):7–26. - PubMed
-
- Deutsch E, Maggiorella L, Eschwege P, Bourhis J, Soria JC, Abdulkarim B. Environmental, genetic, and molecular features of prostate cancer. Lancet Oncol. 2004;5(5):303–13. - PubMed
-
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240(4850):324–6. - PubMed
-
- Hirawat S, Budman DR, Kreis W. The androgen receptor: structure, mutations, and antiandrogens. Cancer Invest. 2003;21(3):400–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

