The HER4 cytoplasmic domain, but not its C terminus, inhibits mammary cell proliferation
- PMID: 17505063
- PMCID: PMC2917064
- DOI: 10.1210/me.2006-0101
The HER4 cytoplasmic domain, but not its C terminus, inhibits mammary cell proliferation
Abstract
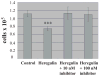
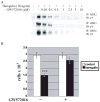
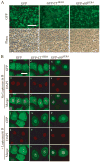
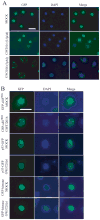
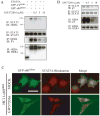
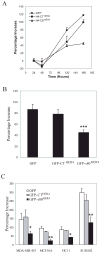
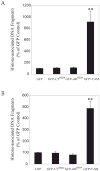
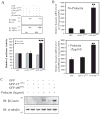
Unlike the proliferative action of other epidermal growth factor (EGF) receptor family members, HER4/ErbB4 is often associated with growth-inhibitory and differentiation signaling. These actions may involve HER4 two-step proteolytic processing by intramembraneous gamma-secretase, releasing the soluble, intracellular 80-kDa HER4 cytoplasmic domain, s80HER4. We demonstrate that pharmacological inhibition of either gamma-secretase activity or HER4 tyrosine kinase activity blocked heregulin-dependent growth inhibition of SUM44 breast cancer cells. We next generated breast cell lines stably expressing GFP-s80HER4 [green fluorescent protein (GFP) fused to the N terminus of the HER4 cytoplasmic domain, residues 676-1308], GFP-CT(HER4) (GFP fused to N terminus of the HER4 C-terminus distal to the tyrosine kinase domain, residues 989-1308), or GFP alone. Both GFP-s80HER4 and GFP-CTHER4 were found in the nucleus, but GFP-s80HER4 accumulated to a greater extent and sustained its nuclear localization. s80HER4 was constitutively tyrosine phosphorylated, and treatment of cells with a specific HER family tyrosine kinase inhibitor 1) blocked tyrosine phosphorylation; 2) markedly diminished GFP-s80HER4 nuclear localization; and 3) reduced signal transducer and activator of transcription (STAT)5A tyrosine phosphorylation and nuclear localization as well as GFP-s80HER4:STAT5A interaction. Multiple normal mammary and breast cancer cell lines, stably expressing GFP-s80HER4 (SUM44, MDA-MB-453, MCF10A, SUM102, and HC11) were growth inhibited compared with the same cell line expressing GFP-CTHER4 or GFP alone. The s80HER4-induced cell number reduction was due to slower growth because rates of apoptosis were equivalent in GFP-, GFP-CTHER4-, and GFP-s80HER4-expressing cells. Lastly, GFP-s80HER4 enhanced differentiation signaling as indicated by increased basal and prolactin-dependent beta-casein expression. These results indicate that surface HER4 tyrosine phosphorylation and ligand-dependent release of s80HER4 are necessary, and s80HER4 signaling is sufficient for HER4-dependent growth inhibition.
Figures

Similar articles
-
Prolactin and ErbB4/HER4 signaling interact via Janus kinase 2 to induce mammary epithelial cell gene expression differentiation.Mol Endocrinol. 2008 Oct;22(10):2307-21. doi: 10.1210/me.2008-0055. Epub 2008 Jul 24. Mol Endocrinol. 2008. PMID: 18653779 Free PMC article.
-
Direct coupling of the HER4 intracellular domain (4ICD) and STAT5A signaling is required to induce mammary epithelial cell differentiation.Biochem Biophys Rep. 2016 Jul 20;7:323-327. doi: 10.1016/j.bbrep.2016.07.015. eCollection 2016 Sep. Biochem Biophys Rep. 2016. PMID: 28955922 Free PMC article.
-
HER4 D-box sequences regulate mitotic progression and degradation of the nuclear HER4 cleavage product s80HER4.Cancer Res. 2007 Jul 15;67(14):6582-90. doi: 10.1158/0008-5472.CAN-06-4145. Cancer Res. 2007. PMID: 17638867 Free PMC article.
-
ErbB4/HER4: role in mammary gland development, differentiation and growth inhibition.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):235-46. doi: 10.1007/s10911-008-9080-x. Epub 2008 Apr 25. J Mammary Gland Biol Neoplasia. 2008. PMID: 18437540 Free PMC article. Review.
-
HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):247-58. doi: 10.1007/s10911-008-9076-6. Epub 2008 May 13. J Mammary Gland Biol Neoplasia. 2008. PMID: 18473151 Free PMC article. Review.
Cited by
-
ErbB2 Is Necessary for ErbB4 Ligands to Stimulate Oncogenic Activities in Models of Human Breast Cancer.Genes Cancer. 2011 Aug;2(8):792-804. doi: 10.1177/1947601911431080. Genes Cancer. 2011. PMID: 22393464 Free PMC article.
-
SHOULD FISH TEST BE PERFORMED TO ALL PATIENTS WITH BREAST CANCER?Med Sci (Turkey). 2013 Jun 1;2(2):539-547. doi: 10.5455/medscience.2013.02.8052. Med Sci (Turkey). 2013. PMID: 24432190 Free PMC article.
-
WW Domain-Containing E3 Ubiquitin Protein Ligase 1: A Self-Disciplined Oncoprotein.Front Cell Dev Biol. 2021 Oct 12;9:757493. doi: 10.3389/fcell.2021.757493. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34712671 Free PMC article. Review.
-
HER Family Receptors are Important Theranostic Biomarkers for Cervical Cancer: Blocking Glucose Metabolism Enhances the Therapeutic Effect of HER Inhibitors.Theranostics. 2017 Jan 15;7(3):717-732. doi: 10.7150/thno.17154. eCollection 2017. Theranostics. 2017. PMID: 28255362 Free PMC article.
-
Aberrant expression of DNA damage response proteins is associated with breast cancer subtype and clinical features.Breast Cancer Res Treat. 2011 Sep;129(2):421-32. doi: 10.1007/s10549-010-1248-6. Epub 2010 Nov 11. Breast Cancer Res Treat. 2011. PMID: 21069451 Free PMC article.
References
-
- Lacenere CJ, Sternberg PW. Regulation of EGF receptor signaling in the fruitfly D. melanogaster and the nematode C. elegans. Breast Dis. 2000;11:19–30. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. - PubMed
-
- Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

