The priming effect of C5a on monocytes is predominantly mediated by the p38 MAPK pathway
- PMID: 17505301
- PMCID: PMC6014696
- DOI: 10.1097/SHK.0b013e31802fa0bd
The priming effect of C5a on monocytes is predominantly mediated by the p38 MAPK pathway
Erratum in
-
The Priming Effect of C5a on Monocytes is Predominantly Mediated by the p38 MAPK Pathway.Shock. 2018 Jul;50(1):127. doi: 10.1097/SHK.0000000000001167. Shock. 2018. PMID: 29677088 Free PMC article. No abstract available.
Abstract
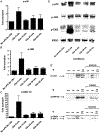
The dysregulation of the inflammatory response after trauma leads to significant morbidity and mortality. Monocytes and macrophages play a central role in the orchestration of the inflammatory response after injury. Serum interleukin-6 (IL-6) concentration correlates with poor outcomes after injury. Tumor necrosis factor-alpha (TNF-alpha) is a proinflammatory cytokine that plays a crucial role in the pathogenesis of multiple organ dysfunction syndrome. Furthermore, in the presence of C5a, monocytes and macrophages have potentiated responses, but the mechanisms underlying this response remain largely unknown. Peripheral blood mononuclear cells (PBMCs) were isolated from healthy volunteers and pretreated with C5a (100 ng/mL) for 1 h before adding lipopolysaccharide (LPS) (10 ng/mL) for up to 20 h. Inhibitors for the mitogen-activated protein kinases (MAPKs) were added 1 h before adding C5a. C5a primes monocytes for LPS-induced IL-6 and TNF-alpha production. Treatment of PBMCs with C5a leads to a rapid activation of the 3 MAPK pathways. SP600125 (inhibitor of c-Jun NH2-terminal kinase MAPK) and PD98059 (inhibitor of extracellular signal-regulated kinase MAPK) did not affect the C5a priming of the LPS-induced IL-6 and TNF-alpha production, whereas SB203580, a specific inhibitor of p38 MAPK, did suppress the C5a priming effect. These results demonstrate that C5a primes adherent PBMCs and modulates LPS-induced IL-6 and TNF-alpha production. Results from extracellular signal-regulated kinase and c-Jun NH2-terminal kinase MAPK blockade suggest that these signaling pathways have minimal or no role in reprogramming LPS-mediated IL-6 and TNF-alpha production. On the contrary, in PBMCs, C5a activates the p38 cascade, and this pathway plays a major role in the C5a enhancement of LPS-induced IL-6 and TNF-alpha production.
Figures


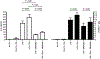
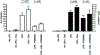

References
-
- Murphy TJ, Paterson HM, Mannick JA, Lederer JA: Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol 75:400–407, 2004. - PubMed
-
- Akira S, Takeda K: Toll-like receptor signalling. Nat Rev Immunol 4:499–511, 2004. - PubMed
-
- Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA: Increased plasma levels of interleukin-6 in sepsis. Blood 74:1704–1710, 1989. - PubMed
-
- Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Bruckner UB: Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg 135:291–295, 2000. - PubMed
-
- Giannoudis PV, Hildebrand F, Pape HC: Inflammatory serum markers in patients with multiple trauma. Can they predict outcome? J Bone Joint Surg Br 86:313–323, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

