Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions
- PMID: 17505518
- PMCID: PMC1868908
- DOI: 10.1038/sj.emboj.7601696
Agrobacterium ParA/MinD-like VirC1 spatially coordinates early conjugative DNA transfer reactions
Abstract
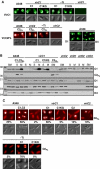
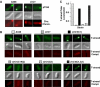
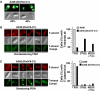
Agrobacterium tumefaciens translocates T-DNA through a polar VirB/D4 type IV secretion (T4S) system. VirC1, a factor required for efficient T-DNA transfer, bears a deviant Walker A and other sequence motifs characteristic of ParA and MinD ATPases. Here, we show that VirC1 promotes conjugative T-DNA transfer by stimulating generation of multiple copies per cell of the T-DNA substrate (T-complex) through pairwise interactions with the processing factors VirD2 relaxase, VirC2, and VirD1. VirC1 also associates with the polar membrane and recruits T-complexes to cell poles, the site of VirB/D4 T4S machine assembly. VirC1 Walker A mutations abrogate T-complex generation and polar recruitment, whereas the native protein recruits T-complexes to cell poles independently of other polar processing factors (VirC2, VirD1) or T4S components (VirD4 substrate receptor, VirB channel subunits). We propose that A. tumefaciens has appropriated a progenitor ParA/MinD-like ATPase to promote conjugative DNA transfer by: (i) nucleating relaxosome assembly at oriT-like T-DNA border sequences and (ii) spatially positioning the transfer intermediate at the cell pole to coordinate substrate-T4S channel docking.
Figures
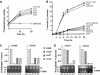

Similar articles
-
Expression of Agrobacterium nopaline-specific VirD1, VirD2, and VirC1 proteins and their requirement for T-strand production in E. coli.Mol Plant Microbe Interact. 1989 Mar-Apr;2(2):43-52. doi: 10.1094/mpmi-2-043. Mol Plant Microbe Interact. 1989. PMID: 2520160
-
DNA substrate-induced activation of the Agrobacterium VirB/VirD4 type IV secretion system.J Bacteriol. 2013 Jun;195(11):2691-704. doi: 10.1128/JB.00114-13. Epub 2013 Apr 5. J Bacteriol. 2013. PMID: 23564169 Free PMC article.
-
Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion.Mol Microbiol. 2004 Dec;54(5):1199-211. doi: 10.1111/j.1365-2958.2004.04345.x. Mol Microbiol. 2004. PMID: 15554962 Free PMC article.
-
Promiscuous DNA transfer system of Agrobacterium tumefaciens: role of the virB operon in sex pilus assembly and synthesis.Mol Microbiol. 1994 Apr;12(1):17-22. doi: 10.1111/j.1365-2958.1994.tb00990.x. Mol Microbiol. 1994. PMID: 7914664 Review.
-
Biogenesis, architecture, and function of bacterial type IV secretion systems.Annu Rev Microbiol. 2005;59:451-85. doi: 10.1146/annurev.micro.58.030603.123630. Annu Rev Microbiol. 2005. PMID: 16153176 Free PMC article. Review.
Cited by
-
Quantitative image analysis and modeling indicate the Agrobacterium tumefaciens type IV secretion system is organized in a periodic pattern of foci.PLoS One. 2012;7(7):e42219. doi: 10.1371/journal.pone.0042219. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860087 Free PMC article.
-
Efficient gene transfer in bacterial cell chains.mBio. 2011 Mar 15;2(2):e00027-11. doi: 10.1128/mBio.00027-11. Print 2011. mBio. 2011. PMID: 21406598 Free PMC article.
-
Mechanism and structure of the bacterial type IV secretion systems.Biochim Biophys Acta. 2014 Aug;1843(8):1578-91. doi: 10.1016/j.bbamcr.2013.12.019. Epub 2014 Jan 2. Biochim Biophys Acta. 2014. PMID: 24389247 Free PMC article. Review.
-
Involvement of the azorhizobial chromosome partition gene (parA) in the onset of bacteroid differentiation during Sesbania rostrata stem nodule development.Appl Environ Microbiol. 2011 Jul;77(13):4371-82. doi: 10.1128/AEM.02327-10. Epub 2011 May 13. Appl Environ Microbiol. 2011. PMID: 21571889 Free PMC article.
-
Carboxysome Mispositioning Alters Growth, Morphology, and Rubisco Level of the Cyanobacterium Synechococcus elongatus PCC 7942.mBio. 2021 Aug 31;12(4):e0269620. doi: 10.1128/mBio.02696-20. Epub 2021 Aug 3. mBio. 2021. PMID: 34340540 Free PMC article.
References
-
- Bignell C, Thomas CM (2001) The bacterial ParA-ParB partitioning proteins. J Biotechnol 91: 1–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

