LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome
- PMID: 17505534
- PMCID: PMC1864997
- DOI: 10.1371/journal.pone.0000448
LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome
Abstract
Background: The multifunctional receptor LRP1 controls expression, activity and trafficking of the PDGF receptor-beta in vascular smooth muscle cells (VSMC). LRP1 is also a receptor for TGFbeta1 and is required for TGFbeta mediated inhibition of cell proliferation.
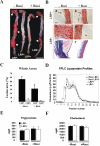
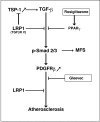
Methods and principal findings: We show that loss of LRP1 in VSMC (smLRP(-)) in vivo results in a Marfan-like syndrome with nuclear accumulation of phosphorylated Smad2/3, disruption of elastic layers, tortuous aorta, and increased expression of the TGFbeta target genes thrombospondin-1 (TSP1) and PDGFRbeta in the vascular wall. Treatment of smLRP1(-) animals with the PPARgamma agonist rosiglitazone abolished nuclear pSmad accumulation, reversed the Marfan-like phenotype, and markedly reduced smooth muscle proliferation, fibrosis and atherosclerosis independent of plasma cholesterol levels.
Conclusions and significance: Our findings are consistent with an activation of TGFbeta signals in the LRP1-deficient vascular wall. LRP1 may function as an integrator of proliferative and anti-proliferative signals that control physiological mechanisms common to the pathogenesis of Marfan syndrome and atherosclerosis, and this is essential for maintaining vascular wall integrity.
Conflict of interest statement
Figures



References
-
- Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. - PubMed
-
- Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, et al. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low Density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. - PubMed
-
- Takayama Y, May P, Anderson RG, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor beta (PDGFR beta). J Biol Chem. 2005;280:18504–18510. - PubMed
-
- Boileau C, Jondeau G, Mizuguchi T, Matsumoto N. Molecular genetics of Marfan syndrome. Curr Opin Cardiol. 2005;20:194–200. - PubMed
-
- Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet. 2005;139:4–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

