Human Maf1 negatively regulates RNA polymerase III transcription via the TFIIB family members Brf1 and Brf2
- PMID: 17505538
- PMCID: PMC1865091
- DOI: 10.7150/ijbs.3.292
Human Maf1 negatively regulates RNA polymerase III transcription via the TFIIB family members Brf1 and Brf2
Abstract
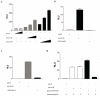
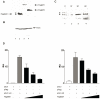
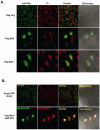
RNA polymerase III (RNA pol III) transcribes many of the small structural RNA molecules involved in processing and translation, thereby regulating the growth rate of a cell. Initiation of pol III transcription requires the evolutionarily conserved pol III initiation factor TFIIIB. TFIIIB is the molecular target of regulation by tumor suppressors, including p53, RB and the RB-related pocket proteins. However, our understanding of negative regulation of human TFIIIB-mediated transcription by other proteins is limited. In this study we characterize a RNA pol III luciferase assay and further demonstrate in vivo that a human homolog of yeast Maf1 represses RNA pol III transcription. Additionally, we show that Maf1 repression of RNA pol III transcription occurs via TFIIIB, specifically through the TFIIB family members Brf1 and Brf2.
Conflict of interest statement
Conflict of Interests: The authors have declared that no conflicts of interests exist.
Figures

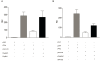

Similar articles
-
RNA polymerase III transcription in cancer: the BRF2 connection.Mol Cancer. 2011 Apr 25;10:47. doi: 10.1186/1476-4598-10-47. Mol Cancer. 2011. PMID: 21518452 Free PMC article. Review.
-
Inhibition of RNA polymerase III transcription by BRCA1.J Mol Biol. 2009 Apr 3;387(3):523-31. doi: 10.1016/j.jmb.2009.02.008. Epub 2009 Feb 11. J Mol Biol. 2009. PMID: 19361418
-
Structure-function analysis of the human TFIIB-related factor II protein reveals an essential role for the C-terminal domain in RNA polymerase III transcription.Mol Cell Biol. 2005 Nov;25(21):9406-18. doi: 10.1128/MCB.25.21.9406-9418.2005. Mol Cell Biol. 2005. PMID: 16227591 Free PMC article.
-
Differential expression of the TFIIIB subunits Brf1 and Brf2 in cancer cells.BMC Mol Biol. 2008 Aug 12;9:74. doi: 10.1186/1471-2199-9-74. BMC Mol Biol. 2008. PMID: 18700021 Free PMC article.
-
Regulation of tRNA synthesis by the general transcription factors of RNA polymerase III - TFIIIB and TFIIIC, and by the MAF1 protein.Biochim Biophys Acta Gene Regul Mech. 2018 Apr;1861(4):320-329. doi: 10.1016/j.bbagrm.2018.01.011. Epub 2018 Feb 6. Biochim Biophys Acta Gene Regul Mech. 2018. PMID: 29378333 Review.
Cited by
-
Gender Specific Differences in RNA Polymerase III Transcription.J Carcinog Mutagen. 2016 Feb;7(1):251. doi: 10.4172/2157-2518.1000251. Epub 2016 Jan 29. J Carcinog Mutagen. 2016. PMID: 27158556 Free PMC article.
-
Full repression of RNA polymerase III transcription requires interaction between two domains of its negative regulator Maf1.J Biol Chem. 2010 Nov 12;285(46):35719-27. doi: 10.1074/jbc.M110.125286. Epub 2010 Sep 3. J Biol Chem. 2010. PMID: 20817737 Free PMC article.
-
Riddle of the Sphinx: Emerging Role of Transfer RNAs in Human Cancer.Front Pharmacol. 2021 Dec 15;12:794986. doi: 10.3389/fphar.2021.794986. eCollection 2021. Front Pharmacol. 2021. PMID: 34975491 Free PMC article. Review.
-
Maf1 regulation: a model of signal transduction inside the nucleus.Nucleus. 2010 Mar-Apr;1(2):162-5. doi: 10.4161/nucl.1.2.11179. Epub 2010 Jan 7. Nucleus. 2010. PMID: 21326948 Free PMC article. Review.
-
RNA polymerase III transcription in cancer: the BRF2 connection.Mol Cancer. 2011 Apr 25;10:47. doi: 10.1186/1476-4598-10-47. Mol Cancer. 2011. PMID: 21518452 Free PMC article. Review.
References
-
- Roeder R.G. Nuclear RNA polymerases: role of general initiation factors and cofactors in eukaryotic transcription. Methods Enzymol. 1996;273:165–71. - PubMed
-
- Schramm L, Hernandez N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 2002;16(20):2593–620. - PubMed
-
- White R.J. RNA polymerases I and III, growth control and cancer. Nat Rev Mol Cell Biol. 2005;6(1):69–78. - PubMed
-
- Gabrielsen O.S, Sentenac A. RNA polymerase III (C) and its transcription factors. Trends Biochem Sci. 1991;16(11):412–6. - PubMed
-
- Desai N et al. Two steps in Maf1-dependent repression of transcription by RNA polymerase III. J Biol Chem. 2005;280(8):6455–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

