BRCA1 may modulate neuronal cell cycle re-entry in Alzheimer disease
- PMID: 17505559
- PMCID: PMC1868658
- DOI: 10.7150/ijms.4.140
BRCA1 may modulate neuronal cell cycle re-entry in Alzheimer disease
Abstract
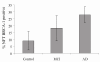
In Alzheimer disease, neuronal degeneration and the presence of neurofibrillary tangles correlate with the severity of cognitive decline. Neurofibrillary tangles contain the antigenic profile of many cell cycle markers, reflecting a re-entry into the cell cycle by affected neurons. However, while such a cell cycle re-entry phenotype is an early and consistent feature of Alzheimer disease, the mechanisms responsible for neuronal cell cycle are unclear. In this regard, given that a dysregulated cell cycle is a characteristic of cancer, we speculated that alterations in oncogenic proteins may play a role in neurodegeneration. To this end, in this study, we examined brain tissue from cases of Alzheimer disease for the presence of BRCA1, a known regulator of cell cycle, and found intense and specific localization of BRCA1 to neurofibrillary tangles, a hallmark lesion of the disease. Analysis of clinically normal aged brain tissue revealed systematically less BRCA1, and surprisingly in many cases with apparent phosphorylated tau-positive neurofibrillary tangles, BRCA1 was absent, yet BRCA1 was present in all cases of Alzheimer disease. These findings not only further define the cell cycle reentry phenotype in Alzheimer disease but also indicate that the neurofibrillary tangles which define Alzheimer disease may have a different genesis from the neurofibrillary tangles of normal aging.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures

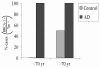


References
-
- Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987;235:1641–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

