Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts
- PMID: 17505910
- PMCID: PMC2117338
- DOI: 10.1007/s11120-007-9181-1
Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts
Abstract
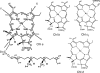
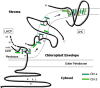
Chlorophyll (Chl) b serves an essential function in accumulation of light-harvesting complexes (LHCs) in plants. In this article, this role of Chl b is explored by considering the properties of Chls and the ligands with which they interact in the complexes. The overall properties of the Chls, not only their spectral features, are altered as consequences of chemical modifications on the periphery of the molecules. Important modifications are introduction of oxygen atoms at specific locations and reduction or desaturation of sidechains. These modifications influence formation of coordination bonds by which the central Mg atom, the Lewis acid, of Chl molecules interacts with amino acid sidechains, as the Lewis base, in proteins. Chl a is a versatile Lewis acid and interacts principally with imidazole groups but also with sidechain amides and water. The 7-formyl group on Chl b withdraws electron density toward the periphery of the molecule and consequently the positive Mg is less shielded by the molecular electron cloud than in Chl a. Chl b thus tends to form electrostatic bonds with Lewis bases with a fixed dipole, such as water and, in particular, peptide backbone carbonyl groups. The coordination bonds are enhanced by H-bonds between the protein and the 7-formyl group. These additional strong interactions with Chl b are necessary to achieve assembly of stable LHCs.
Figures


Similar articles
-
A potential role of chlorophylls b and c in assembly of light-harvesting complexes.FEBS Lett. 2001 Jan 26;489(1):1-3. doi: 10.1016/s0014-5793(00)02410-8. FEBS Lett. 2001. PMID: 11231002
-
The role of chlorophyll b in photosynthesis: hypothesis.BMC Plant Biol. 2001;1:2. doi: 10.1186/1471-2229-1-2. Epub 2001 Oct 17. BMC Plant Biol. 2001. PMID: 11710960 Free PMC article.
-
Influence of structure on binding of chlorophylls to peptide ligands.J Am Chem Soc. 2005 Feb 23;127(7):2052-3. doi: 10.1021/ja043462b. J Am Chem Soc. 2005. PMID: 15713076
-
Water soluble chlorophyll binding protein of higher plants: a most suitable model system for basic analyses of pigment-pigment and pigment-protein interactions in chlorophyll protein complexes.J Plant Physiol. 2011 Aug 15;168(12):1462-72. doi: 10.1016/j.jplph.2010.12.005. Epub 2011 Jan 21. J Plant Physiol. 2011. PMID: 21256622 Review.
-
Organization of chlorophyll biosynthesis and insertion of chlorophyll into the chlorophyll-binding proteins in chloroplasts.Photosynth Res. 2015 Dec;126(2-3):189-202. doi: 10.1007/s11120-015-0154-5. Epub 2015 May 9. Photosynth Res. 2015. PMID: 25957270 Review.
Cited by
-
Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives.Polymers (Basel). 2023 Dec 28;16(1):101. doi: 10.3390/polym16010101. Polymers (Basel). 2023. PMID: 38201766 Free PMC article.
-
Cold acclimation alleviates photosynthetic inhibition and oxidative damage induced by cold stress in citrus seedlings.Plant Signal Behav. 2023 Dec 31;18(1):2285169. doi: 10.1080/15592324.2023.2285169. Epub 2023 Nov 28. Plant Signal Behav. 2023. PMID: 38015652 Free PMC article.
-
Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes.Photosynth Res. 2010 Nov;106(1-2):57-71. doi: 10.1007/s11120-010-9576-2. Epub 2010 Jul 2. Photosynth Res. 2010. PMID: 20596891 Review.
-
Identification of the Optimal Light Harvesting Antenna Size for High-Light Stress Mitigation in Plants.Front Plant Sci. 2020 May 15;11:505. doi: 10.3389/fpls.2020.00505. eCollection 2020. Front Plant Sci. 2020. PMID: 32499795 Free PMC article.
-
Molecular mechanism of SRP-dependent light-harvesting protein transport to the thylakoid membrane in plants.Photosynth Res. 2018 Dec;138(3):303-313. doi: 10.1007/s11120-018-0544-6. Epub 2018 Jun 28. Photosynth Res. 2018. PMID: 29956039 Free PMC article. Review.
References
-
- None
- Abramov YuA, Volkov AV, Coppens P (1999) On the evaluation of molecule dipole moments from multipole refinement of X-ray diffraction data. Chem Phys Lett 311:81–86
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '3924407', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/3924407/'}]}
- Adams GA, Rose JK (1985) Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell 41:1007–1015 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16228548', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16228548/'}]}
- Akiyama M, Miyashita H, Kise H, Watanabe T, Mimuro M, Miyachi S, Kobayashi M (2002) Quest for minor but key chlorophyll molecules in photosynthetic reaction centers—unusual pigment composition in the reaction centers of the chlorophyll d-dominated cyanobacterium Acaryochloris marina. Photosynth Res 74:97–107 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12047194', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12047194/'}]}
- Antoine R, Compagnon I, Rayane D, Broyer M, Dugourd P, Breaux G, Hagemeister FC, Pippen D, Hudgins RR, Jarrold MF (2002) Electric susceptibility of unsolvated glycine-based peptides. J Am Chem Soc 124:6737–6741 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '15917498', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15917498/'}]}
- Bachvaroff TR, Puerta MVS, Delwiche CF (2005) Chlorophyll c-containing plastid relationships based on analyses of a multigene data set with all four chromalveolate lineages. Mol Biol Evol 22:1772–1782 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

