Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition
- PMID: 17506520
- PMCID: PMC2747370
- DOI: 10.1021/bp070011x
Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition
Abstract
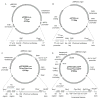
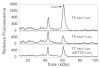
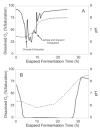
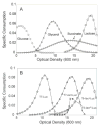
The auto-induction method of protein expression in E. coli is based on diauxic growth resulting from dynamic function of lac operon regulatory elements (lacO and LacI) in mixtures of glucose, glycerol, and lactose. The results show that successful execution of auto-induction is strongly dependent on the plasmid promoter and repressor construction, on the oxygenation state of the culture, and on the composition of the auto-induction medium. Thus expression hosts expressing high levels of LacI during aerobic growth exhibit reduced ability to effectively complete the auto-induction process. Manipulation of the promoter to decrease the expression of LacI altered the preference for lactose consumption in a manner that led to increased protein expression and partially relieved the sensitivity of the auto-induction process to the oxygenation state of the culture. Factorial design methods were used to optimize the chemically defined growth medium used for expression of two model proteins, Photinus luciferase and enhanced green fluorescent protein, including variations for production of both unlabeled and selenomethionine-labeled samples. The optimization included studies of the expression from T7 and T7-lacI promoter plasmids and from T5 phage promoter plasmids expressing two levels of LacI. Upon the basis of the analysis of over 500 independent expression results, combinations of optimized expression media and expression plasmids that gave protein yields of greater than 1000 mug/mL of expression culture were identified.
Figures






Similar articles
-
Escherichia coli σ70 promoters allow expression rate control at the cellular level in genome-integrated expression systems.Microb Cell Fact. 2020 Mar 5;19(1):58. doi: 10.1186/s12934-020-01311-6. Microb Cell Fact. 2020. PMID: 32138729 Free PMC article.
-
Natural LacI from E. coli yields faster response and higher level of expression than the LVA-tagged LacI.ACS Synth Biol. 2014 Dec 19;3(12):949-52. doi: 10.1021/sb500031h. ACS Synth Biol. 2014. PMID: 25524095
-
High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction: effect of lac operator.PLoS One. 2013 Oct 23;8(10):e78416. doi: 10.1371/journal.pone.0078416. eCollection 2013. PLoS One. 2013. PMID: 24194930 Free PMC article.
-
Review: optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter.J Ind Microbiol. 1996 Mar;16(3):145-54. doi: 10.1007/BF01569997. J Ind Microbiol. 1996. PMID: 8652113 Review.
-
Exploitation of the Escherichia coli lac operon promoter for controlled recombinant protein production.Biochem Soc Trans. 2019 Apr 30;47(2):755-763. doi: 10.1042/BST20190059. Epub 2019 Apr 10. Biochem Soc Trans. 2019. PMID: 30971435 Review.
Cited by
-
Cascaded processing enables continuous upstream processing with E. coli BL21(DE3).Sci Rep. 2021 Jun 1;11(1):11477. doi: 10.1038/s41598-021-90899-9. Sci Rep. 2021. PMID: 34075099 Free PMC article.
-
Construction of an AI-2 quorum sensing induced heterologous protein expression system in Escherichia coli.PeerJ. 2021 Nov 16;9:e12497. doi: 10.7717/peerj.12497. eCollection 2021. PeerJ. 2021. PMID: 34820206 Free PMC article.
-
Microaerobic fermentation alters lactose metabolism in Escherichia coli.Appl Microbiol Biotechnol. 2020 Jul;104(13):5773-5785. doi: 10.1007/s00253-020-10652-6. Epub 2020 May 15. Appl Microbiol Biotechnol. 2020. PMID: 32409946
-
PKS-NRPS Enzymology and Structural Biology: Considerations in Protein Production.Methods Enzymol. 2018;604:45-88. doi: 10.1016/bs.mie.2018.01.035. Epub 2018 Mar 16. Methods Enzymol. 2018. PMID: 29779664 Free PMC article.
-
The Complex Energy Landscape of the Protein IscU.Biophys J. 2015 Sep 1;109(5):1019-25. doi: 10.1016/j.bpj.2015.07.045. Biophys J. 2015. PMID: 26331259 Free PMC article.
References
-
- Zhang C, Kim SH. Overview of Structural Genomics: From Structure to Function. Curr Opin Chem Biol. 2003;7:28–32. - PubMed
-
- Terwilliger TC. Structural Genomics in North America. Nat Struct Mol Biol. 2000;7(Suppl):935–9. - PubMed
-
- Chandonia JM, Brenner SE. The Impact of Structural Genomics: Expectations and Outcomes. Science. 2006;311:347–51. - PubMed
-
- Becker KF, Metzger V, Hipp S, Hofler H. Clinical Proteomics: New Trends for Protein Microarrays. Curr Med Chem. 2006;13:1831–7. - PubMed
-
- Arcus VL, Lott JS, Johnston JM, Baker EN. The Potential Impact of Structural Genomics on Tuberculosis Drug Discovery. Drug Discovery Today. 2006;11:28–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

