Neuron-glial interactions in blood-brain barrier formation
- PMID: 17506642
- PMCID: PMC2824917
- DOI: 10.1146/annurev.neuro.30.051606.094345
Neuron-glial interactions in blood-brain barrier formation
Abstract
The blood brain barrier (BBB) evolved to preserve the microenvironment of the highly excitable neuronal cells to allow for action potential generation and propagation. Intricate molecular interactions between two main cell types, the neurons and the glial cells, form the underlying basis of the critical functioning of the nervous system across species. In invertebrates, interactions between neurons and glial cells are central in establishing a functional BBB. However, in vertebrates, the BBB formation and function is coordinated by interactions between neurons, glial cells, and endothelial cells. Here we review the neuron-glial interaction-based blood barriers in invertebrates and vertebrates and provide an evolutionary perspective as to how a glial-barrier system in invertebrates evolved into an endothelial barrier system. We also summarize the clinical relevance of the BBB as this protective barrier becomes disadvantageous in the pharmacological treatment of various neurological disorders.
Figures
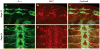
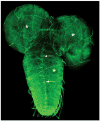




References
-
- Abbott NJ. Comparative physiology of the blood-brain barrier. In: Bradbury MWB, editor. Physiology and Pharmacology of the Blood-Brain-Barrier. Berlin: Springer-Verlag; 1992. pp. 371–96.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

