Naturally occurring and bioengineered apoA-I mutations that inhibit the conversion of discoidal to spherical HDL: the abnormal HDL phenotypes can be corrected by treatment with LCAT
- PMID: 17506726
- PMCID: PMC1948983
- DOI: 10.1042/BJ20070296
Naturally occurring and bioengineered apoA-I mutations that inhibit the conversion of discoidal to spherical HDL: the abnormal HDL phenotypes can be corrected by treatment with LCAT
Abstract
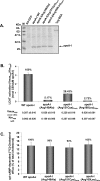
In the present study we have used adenovirus-mediated gene transfer of apoA-I (apolipoprotein A-I) mutants in apoA-I-/- mice to investigate how structural mutations in apoA-I affect the biogenesis and the plasma levels of HDL (high-density lipoprotein). The natural mutants apoA-I(R151C)Paris, apoA-I(R160L)Oslo and the bioengineered mutant apoA-I(R149A) were secreted efficiently from cells in culture. Their capacity to activate LCAT (lecithin:cholesterol acyltransferase) in vitro was greatly reduced, and their ability to promote ABCA1 (ATP-binding cassette transporter A1)-mediated cholesterol efflux was similar to that of WT (wild-type) apoA-I. Gene transfer of the three mutants in apoA-I-/- mice generated aberrant HDL phenotypes. The total plasma cholesterol of mice expressing the apoA-I(R160L)Oslo, apoA-I(R149A) and apoA-I(R151C)Paris mutants was reduced by 78, 59 and 61% and the apoA-I levels were reduced by 68, 64 and 55% respectively, as compared with mice expressing the WT apoA-I. The CE (cholesteryl ester)/TC (total cholesterol) ratio of HDL was decreased and the apoA-I was distributed in the HDL3 region. apoA-I(R160L)Oslo and apoA-I(R149A) promoted the formation of prebeta1 and alpha4-HDL subpopulations and gave a mixture of discoidal and spherical particles. apoA-I(R151C)Paris generated subpopulations of different sizes that migrate between prebeta and alpha-HDL and formed mostly spherical and a few discoidal particles. Simultaneous treatment of mice with adenovirus expressing any of the three mutants and human LCAT normalized plasma apoA-I, HDL cholesterol levels and the CE/TC ratio. It also led to the formation of spherical HDL particles consisting mostly of alpha-HDL subpopulations of larger size. The correction of the aberrant HDL phenotypes by treatment with LCAT suggests a potential therapeutic intervention for HDL abnormalities that result from specific mutations in apoA-I.
Figures



References
-
- Zannis V. I., Kardassis D., Zanni E. E. Genetic mutations affecting human lipoproteins, their receptors and their enzymes. Adv. Hum. Genet. 1993;21:145–319. - PubMed
-
- Chroni A., Liu T., Gorshkova I., Kan H. Y., Uehara Y., von Eckardstein A., Zannis V. I. The central helices of apoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid residues 220-231 of the wild-type apoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J. Biol. Chem. 2003;278:6719–6730. - PubMed
-
- Laccotripe M., Makrides S. C., Jonas A., Zannis V. I. The carboxyl-terminal hydrophobic residues of apolipoprotein A-I affect its rate of phospholipid binding and its association with high density lipoprotein. J. Biol. Chem. 1997;272:17511–17522. - PubMed
-
- Liu T., Krieger M., Kan H. Y., Zannis V. I. The effects of mutations in helices 4 and 6 of apoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

