A pharmacokinetic model for L-carnitine in patients receiving haemodialysis
- PMID: 17506778
- PMCID: PMC2000652
- DOI: 10.1111/j.1365-2125.2007.02926.x
A pharmacokinetic model for L-carnitine in patients receiving haemodialysis
Abstract
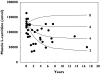
Aims: Patients requiring chronic haemodialysis may develop a secondary carnitine deficiency through dialytic loss of L-carnitine. A previous report has described the plasma concentrations of L-carnitine in 12 such patients under baseline conditions and after L-carnitine administration (20 mg kg(-1)). A three-compartment pharmacokinetic model was developed to describe these data to make inferences about carnitine supplementation in these patients.
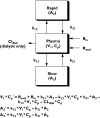
Methods: L-carnitine removal was mediated solely by intermittent haemodialysis, which was incorporated into the model as an experimentally derived dialysis clearance value that was linked to an on-off pulse function. Data were described by a model with a central compartment linked to 'fast'- and 'slow'-equilibrating peripheral compartments.
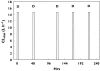
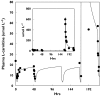
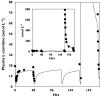
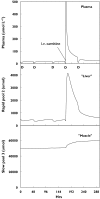
Results: The model adequately described the changing plasma concentrations of endogenous L-carnitine in individual haemodialysis patients. Based on pooled data (mean +/- SD; n = 12), the volume of the central compartment was 10.09 +/- 0.72 l and the transfer rate constants into and out of the slowly equilibrating pool were 0.100 +/- 0.018 h(-1) and 0.00014 +/- 0.00016 h(-1), respectively. The turnover time of L-carnitine in the slow pool (which was assumed to represent muscle) was approximately 300 days. The model was in general agreement with separate data on the measured loss of carnitine from muscle in dialysis patients.
Conclusions: Haemodialysis causes rapid reductions in plasma L-carnitine concentrations with each dialysis session. Plasma concentrations are restored between sessions by redistribution from peripheral compartments. However, during chronic haemodialysis, the ongoing dialytic loss of L-carnitine may lead to a slow depletion of the compound, contributing to a possible secondary deficiency.
Figures
Similar articles
-
Pharmacokinetics of L-carnitine in patients with end-stage renal disease undergoing long-term hemodialysis.Clin Pharmacol Ther. 2000 Sep;68(3):238-49. doi: 10.1067/mcp.2000.108850. Clin Pharmacol Ther. 2000. PMID: 11014405
-
Insulin action on glucose and protein metabolism during L-carnitine supplementation in maintenance haemodialysis patients.Nephrol Dial Transplant. 2008 Mar;23(3):991-7. doi: 10.1093/ndt/gfm664. Epub 2007 Nov 28. Nephrol Dial Transplant. 2008. PMID: 18045815 Clinical Trial.
-
Pharmacokinetics of L-carnitine.Clin Pharmacokinet. 2003;42(11):941-67. doi: 10.2165/00003088-200342110-00002. Clin Pharmacokinet. 2003. PMID: 12908852 Review.
-
Endogenous plasma carnitine pool composition and response to erythropoietin treatment in chronic haemodialysis patients.Nephrol Dial Transplant. 2009 Mar;24(3):990-6. doi: 10.1093/ndt/gfn588. Epub 2008 Nov 5. Nephrol Dial Transplant. 2009. PMID: 18987259
-
Dialysis-related carnitine disorder.Semin Dial. 2006 Jul-Aug;19(4):323-8. doi: 10.1111/j.1525-139X.2006.00180.x. Semin Dial. 2006. PMID: 16893411 Review.
Cited by
-
Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: a randomized controlled trial.Nutrients. 2014 Dec 22;6(12):5992-6004. doi: 10.3390/nu6125992. Nutrients. 2014. PMID: 25533009 Free PMC article. Clinical Trial.
-
Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects.Clin Pharmacokinet. 2012 Sep 1;51(9):553-72. doi: 10.1007/BF03261931. Clin Pharmacokinet. 2012. PMID: 22804748 Review.
-
Association between 4-year all-cause mortality and carnitine profile in maintenance hemodialysis patients.PLoS One. 2018 Aug 22;13(8):e0201591. doi: 10.1371/journal.pone.0201591. eCollection 2018. PLoS One. 2018. PMID: 30133480 Free PMC article.
-
"Exploring benefits of L-carnitine in hemodialysis: promising findings await clinical application".Int Urol Nephrol. 2025 Apr 25. doi: 10.1007/s11255-025-04550-x. Online ahead of print. Int Urol Nephrol. 2025. PMID: 40281379
-
Carnitine Deficiency in Chronic Kidney Disease: Pathophysiology, Clinical Implications, and Therapeutic Perspectives.Nutrients. 2025 Jun 23;17(13):2084. doi: 10.3390/nu17132084. Nutrients. 2025. PMID: 40647189 Free PMC article. Review.
References
-
- Bremer J. Carnitine – metabolism and functions. Physiol Rev. 1983;63:1420–80. - PubMed
-
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann NY Acad Sci. 2004;1033:30–41. - PubMed
-
- Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clin Pharmacokinet. 2003;42:941–67. - PubMed
-
- Ahmad S. L-carnitine in dialysis patients. Semin Dialysis. 2001;14:209–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

