The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects
- PMID: 17506785
- PMCID: PMC2000643
- DOI: 10.1111/j.1365-2125.2007.02899.x
The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects
Abstract
Aims: The novel direct thrombin inhibitor (DTI), dabigatran etexilate (Boehringer Ingelheim Pharma GmbH & Co. KG), shows potential as an oral antithrombotic agent. Two double-blind, randomized trials were undertaken to investigate the pharmacokinetics (PK), pharmacodynamics (PD) and tolerability of orally administered dabigatran etexilate in healthy male subjects.
Methods: Dabigatran etexilate or placebo was administered orally at single doses of 10-400 mg (n = 40) or at multiple doses of 50-400 mg three times daily for 6 days (n = 40). Plasma and urine samples were collected over time to determine the PK profile of dabigatran. PD activity was assessed by its effects on blood coagulation parameters: activated partial thromboplastin time (aPTT), prothrombin time (PT), reported as international normalized ratio (INR), thrombin time (TT), and ecarin clotting time (ECT). All adverse events were recorded.
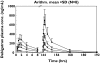
Results: Dabigatran etexilate was rapidly absorbed with peak plasma concentrations of dabigatran reached within 2 h of administration. This was followed by a rapid distribution/elimination phase and a terminal phase, with associated estimated half-lives of 8-10 h and 14-17 h with single and multiple dose administrations, respectively. Dabigatran exhibited linear PK characteristics with dose-proportional increases observed in maximum plasma concentration and area under the curve. Steady-state conditions were reached within 3 days with multiple dosing. The mean apparent volume of distribution during the terminal phase (V(z)/F) of 1860 l (range 1430-2400 l) and the apparent total clearance after oral administration (CL(tot)/F) of 2031 ml min(-1) (range 1480-2430), were dose independent. Time curves for aPTT, INR, TT and ECT paralleled plasma concentration-time curves with values increasing rapidly and in a dose-dependent manner. At the highest dose of 400 mg administered three times daily, maximum prolongations over baseline of 3.1 (aPTT), 3.5 (INR), 29 (TT) and 9.5-fold (ECT) were observed. Dabigatran underwent conjugation with glucuronic acid to form pharmacologically active conjugates that accounted for approximately 20% of total dabigatran in plasma. Overall, variability in PK parameters was low to moderate, with an average interindividual coefficient of variation (CV) of approximately 30% and variability in PD parameters was low, with CV < 10%. Of the four assays, TT and ECT exhibited the greatest sensitivity and precision within the anticipated therapeutic dose range. Bleeding events were few and were mild-to-moderate in intensity, occurring only in the higher, multiple dose groups.
Conclusions: These data suggest that dabigatran etexilate is a promising novel oral DTI with predictable PK and PD characteristics and good tolerability. Further investigation of dabigatran etexilate for the treatment and prophylaxis of patients with arterial and venous thromboembolic disorders, acute coronary syndromes and other medical conditions is warranted.
Figures


Comment in
-
Development of oral anticoagulants.Br J Clin Pharmacol. 2007 Sep;64(3):263-5. doi: 10.1111/j.1365-2125.2007.02898.x. Epub 2007 May 15. Br J Clin Pharmacol. 2007. PMID: 17506786 Free PMC article. No abstract available.
References
-
- Haas S. Oral direct thrombin inhibition: an effective and novel approach for venous thromboembolism. Drugs. 2004;64(Suppl 1):7–16. - PubMed
-
- Hyers TM. Management of venous thromboembolism: past, present and future. Arch Intern Med. 2003;163:759–68. - PubMed
-
- Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Sixth ACCP Consensus Conference on Antithrombotic therapy. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(Suppl):8S–21S. - PubMed
-
- Nutescu EA, Helgason CM, Briller J, Schwertz DW. New blood thinner offers first potential alternative in 50 years: ximelagatran. J Cardiovasc Nurs. 2004;19:374–83. - PubMed
-
- Agnelli G. Clinical potential of oral direct thrombin inhibitors in the prevention and treatment of venous thromboembolism. Drugs. 2004;64(Suppl 1):47–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

