Chronic intranasal administration of mould spores or extracts to unsensitized mice leads to lung allergic inflammation, hyper-reactivity and remodelling
- PMID: 17506853
- PMCID: PMC2265999
- DOI: 10.1111/j.1365-2567.2007.02636.x
Chronic intranasal administration of mould spores or extracts to unsensitized mice leads to lung allergic inflammation, hyper-reactivity and remodelling
Abstract
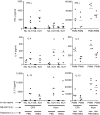
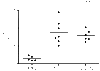
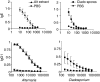
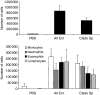
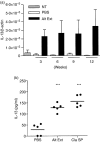
Allergic asthma is a serious multifaceted disease characterized by eosinophil-rich airway inflammation, airway hyperreactivity and airway wall modifications known as remodelling. We previously demonstrated that the spores of two allergenic moulds, Alternaria alternata and Cladosporium herbarum, were potent inducers of immunoglobulin E (IgE) production. Moreover, mice sensitized by two intraperitoneal injections before intranasal challenge with A. alternata or C. herbarum spores developed an allergic lung inflammation and hyperreactivity. Here we report on the effect of chronic intranasal administration of C. herbarum spores or A. alternata extracts to unsensitized BALB/c mice. Our results demonstrate that this chronic treatment led to an increase of total serum IgE and the appearance of specific IgE and IgG1. Total cell number in bronchoalveolar lavage fluid from treated mice was highly increased compared to phosphate-buffered-saline-treated mice because of the accumulation of macrophages, neutrophils, lymphocytes and eosinophils. Airway hyperreactivity appeared after 3 weeks (extract) and 7 weeks (spores) and was maintained during the whole treatment. Increased interleukin-13 mRNA expression in the lungs and T helper type 2 cytokines (interleukin-4, -5, -6 and -13) and transforming growth factor-beta secretion in bronchoalveolar lavage fluid were also observed. Lung hydroxyproline and fibronectin contents indicated increased fibrosis in mice treated with mould allergen. These observations were confirmed by histological analysis demonstrating airway wall remodelling and strong mucus production. These observations show that this model, using chronic intranasal administration of relevant particulate allergens, is an interesting tool for the study of mechanisms leading to allergic pulmonary diseases and lung remodelling.
Figures
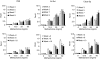
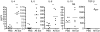
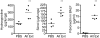

Similar articles
-
A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds.Clin Exp Immunol. 2005 Feb;139(2):179-88. doi: 10.1111/j.1365-2249.2004.02679.x. Clin Exp Immunol. 2005. PMID: 15654816 Free PMC article.
-
4-1 BB stimulation inhibits allergen-specific immunoglobulin E production and airway hyper-reactivity but partially suppresses bronchial eosinophilic inflammation in a mouse asthma model.Clin Exp Allergy. 2006 Mar;36(3):377-85. doi: 10.1111/j.1365-2222.2006.02445.x. Clin Exp Allergy. 2006. PMID: 16499650
-
Comparison of acute inflammatory and chronic structural asthma-like responses between C57BL/6 and BALB/c mice.Int Arch Allergy Immunol. 2009;149(3):195-207. doi: 10.1159/000199715. Epub 2009 Feb 12. Int Arch Allergy Immunol. 2009. PMID: 19218812
-
Potential of immunoglobulin A to prevent allergic asthma.Clin Dev Immunol. 2013;2013:542091. doi: 10.1155/2013/542091. Epub 2013 Apr 11. Clin Dev Immunol. 2013. PMID: 23690823 Free PMC article. Review.
-
The alveolar macrophages in asthma: a double-edged sword.Mucosal Immunol. 2012 Nov;5(6):605-9. doi: 10.1038/mi.2012.74. Epub 2012 Aug 22. Mucosal Immunol. 2012. PMID: 22910216 Review.
Cited by
-
Genetic susceptibility to allergic bronchopulmonary aspergillosis in asthma: a genetic association study.Allergy Asthma Clin Immunol. 2016 Sep 27;12:47. doi: 10.1186/s13223-016-0152-y. eCollection 2016. Allergy Asthma Clin Immunol. 2016. PMID: 27708669 Free PMC article.
-
Different Airway Inflammatory Phenotypes Correlate with Specific Fungal and Bacterial Microbiota in Asthma and Chronic Obstructive Pulmonary Disease.J Immunol Res. 2022 Mar 11;2022:2177884. doi: 10.1155/2022/2177884. eCollection 2022. J Immunol Res. 2022. PMID: 35310604 Free PMC article.
-
Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi.Mucosal Immunol. 2009 Nov;2(6):504-17. doi: 10.1038/mi.2009.102. Epub 2009 Aug 26. Mucosal Immunol. 2009. PMID: 19710638 Free PMC article.
-
Antibodies generated against conserved antigens expressed by bacteria and allergen-bearing fungi suppress airway disease.J Immunol. 2012 Sep 1;189(5):2246-56. doi: 10.4049/jimmunol.1200702. Epub 2012 Jul 25. J Immunol. 2012. PMID: 22837487 Free PMC article.
-
Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy.Mucosal Immunol. 2014 Sep;7(5):1068-78. doi: 10.1038/mi.2013.123. Epub 2014 Feb 5. Mucosal Immunol. 2014. PMID: 24496315 Free PMC article.
References
-
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–20. - PubMed
-
- Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51:323–82. - PubMed
-
- Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344:350–62. - PubMed
-
- Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

