A mechanism for the sharp transition of morphogen gradient interpretation in Xenopus
- PMID: 17506890
- PMCID: PMC1885807
- DOI: 10.1186/1471-213X-7-47
A mechanism for the sharp transition of morphogen gradient interpretation in Xenopus
Abstract
Background: One way in which positional information is established during embryonic development is through the graded distribution of diffusible morphogens. Unfortunately, little is known about how cells interpret different concentrations of morphogen to activate different genes or how thresholds are generated in a morphogen gradient.
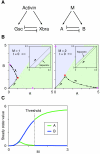
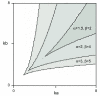
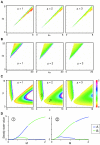
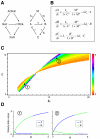
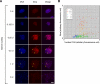
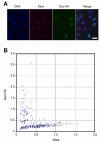
Results: Here we show that the concentration-dependent induction of the T-box transcription factor Brachyury (Xbra) and the homeobox-containing gene Goosecoid (Gsc) by activin in Xenopus can be explained by the dynamics of a simple network consisting of three elements with a mutual negative feedback motif that can function to convert a graded signal (activin) into a binary output (Xbra on and Gsc off, or vice versa). Importantly, such a system can display sharp thresholds. Consistent with the predictions of our model, Xenopus ectodermal cells display a binary response at the single cell level after treatment with activin.
Conclusion: This kind of simple network with mutual negative feedback might provide a general mechanism for selective gene activation in response to different levels of a single external signal. It provides a mechanism by which a sharp boundary might be created between domains of different cell types in response to a morphogen gradient.
Figures

Similar articles
-
Two-dimensional morphogen gradient in Xenopus: boundary formation and real-time transduction response.Dev Dyn. 2006 Dec;235(12):3189-98. doi: 10.1002/dvdy.20963. Dev Dyn. 2006. PMID: 17029288
-
Morphogen gradients, positional information, and Xenopus: interplay of theory and experiment.Dev Dyn. 2002 Dec;225(4):392-408. doi: 10.1002/dvdy.10170. Dev Dyn. 2002. PMID: 12454918 Review.
-
Formation of a functional morphogen gradient by a passive process in tissue from the early Xenopus embryo.Int J Dev Biol. 2001;45(1):199-207. Int J Dev Biol. 2001. PMID: 11291847
-
Single cells can sense their position in a morphogen gradient.Development. 1999 Dec;126(23):5309-17. doi: 10.1242/dev.126.23.5309. Development. 1999. PMID: 10556056
-
Scaling of morphogen gradients.Curr Opin Genet Dev. 2011 Dec;21(6):704-10. doi: 10.1016/j.gde.2011.07.011. Epub 2011 Aug 26. Curr Opin Genet Dev. 2011. PMID: 21873045 Review.
Cited by
-
Design principles of stripe-forming motifs: the role of positive feedback.Sci Rep. 2014 May 16;4:5003. doi: 10.1038/srep05003. Sci Rep. 2014. PMID: 24830352 Free PMC article.
-
Implications of diffusion and time-varying morphogen gradients for the dynamic positioning and precision of bistable gene expression boundaries.PLoS Comput Biol. 2021 Jun 1;17(6):e1008589. doi: 10.1371/journal.pcbi.1008589. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34061823 Free PMC article.
-
Multistable decision switches for flexible control of epigenetic differentiation.PLoS Comput Biol. 2008 Nov;4(11):e1000235. doi: 10.1371/journal.pcbi.1000235. Epub 2008 Nov 28. PLoS Comput Biol. 2008. PMID: 19043543 Free PMC article.
-
Polarization and migration in the zebrafish posterior lateral line system.PLoS Comput Biol. 2017 Apr 3;13(4):e1005451. doi: 10.1371/journal.pcbi.1005451. eCollection 2017 Apr. PLoS Comput Biol. 2017. PMID: 28369079 Free PMC article.
-
Controlling spatiotemporal pattern formation in a concentration gradient with a synthetic toggle switch.Mol Syst Biol. 2020 Jun;16(6):e9361. doi: 10.15252/msb.20199361. Mol Syst Biol. 2020. PMID: 32529808 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

