Oral administration of live virus protects susceptible mice from developing Theiler's virus-induced demyelinating disease
- PMID: 17507073
- PMCID: PMC2025699
- DOI: 10.1016/j.virol.2007.04.017
Oral administration of live virus protects susceptible mice from developing Theiler's virus-induced demyelinating disease
Abstract
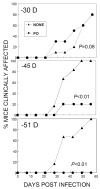
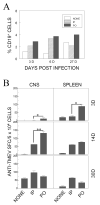
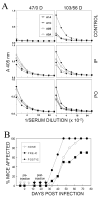
Intracerebral infection of susceptible mouse strains with Theiler's murine encephalomyelitis virus (TMEV) results in an immune-mediated demyelinating disease similar to human multiple sclerosis. TMEV infection is widely spread via fecal-oral routes among wild mouse populations, yet these infected mice rarely develop clinical disease. Oral vaccination has often been used to protect the host against many different infectious agents, although the underlying protective mechanism of prior oral exposure is still unknown. To understand the mechanisms involved in protection from demyelinating disease following previous oral infection, immune parameters and disease progression of mice perorally infected with TMEV were compared with those of mice immunized intraperitoneally following intracerebral infection. Mice infected perorally, but not intraperitoneally, prior to CNS viral infection showed lower chronic viral persistence in the CNS and reduced TMEV-induced demyelinating disease. However, a prolonged period of post-oral infection was necessary for effective protection. Mice orally pre-exposed to the virus displayed markedly elevated levels of antibody response to TMEV in the serum, although T cell responses to TMEV in the periphery were not significantly different between perorally and intraperitoneally immunized mice. In addition, orally vaccinated mice showed higher levels of early CNS-infiltration of B cells producing anti-TMEV antibody as well as virus-specific CD4(+) and CD8(+) T cells in the CNS compared to intraperitoneally immunized mice. Therefore, the generation of a sufficient level of protective immune responses appears to require a prolonged time period to confer protection from TMEV-induced demyelinating disease.
Figures




Similar articles
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
-
CD8-deficient SJL mice display enhanced susceptibility to Theiler's virus infection and increased demyelinating pathology.J Neurovirol. 2001 Oct;7(5):409-20. doi: 10.1080/135502801753170264. J Neurovirol. 2001. PMID: 11582513 Free PMC article.
-
A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1(233-244).J Immunol. 1994 Nov 15;153(10):4508-19. J Immunol. 1994. PMID: 7525707
-
IFNγ influences type I interferon response and susceptibility to Theiler's virus-induced demyelinating disease.Viral Immunol. 2013 Aug;26(4):223-38. doi: 10.1089/vim.2013.0004. Epub 2013 Jul 5. Viral Immunol. 2013. PMID: 23829778 Free PMC article.
-
Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis.J Neuroimmune Pharmacol. 2010 Sep;5(3):355-69. doi: 10.1007/s11481-009-9179-x. Epub 2009 Nov 6. J Neuroimmune Pharmacol. 2010. PMID: 19894121 Free PMC article. Review.
Cited by
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
-
Isolation, Identification, and Evaluation of the Pathogenicity of a Porcine Enterovirus G Isolated From China.Front Vet Sci. 2021 Jul 22;8:712679. doi: 10.3389/fvets.2021.712679. eCollection 2021. Front Vet Sci. 2021. PMID: 34368288 Free PMC article.
-
Virus infection, antiviral immunity, and autoimmunity.Immunol Rev. 2013 Sep;255(1):197-209. doi: 10.1111/imr.12091. Immunol Rev. 2013. PMID: 23947356 Free PMC article. Review.
-
Anticapsid immunity level, not viral persistence level, correlates with the progression of Theiler's virus-induced demyelinating disease in viral P1-transgenic mice.J Virol. 2008 Jun;82(11):5606-17. doi: 10.1128/JVI.02442-07. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353953 Free PMC article.
References
-
- Burton D. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–13. - PubMed
-
- Cash E, Bandeira A, Chirinian S, Brahic M. Characterization of B lymphocytes present in the demyelinating lesions induced by Theiler’s virus [published erratum appears in J Immunol 1989 Sep 15;143(6):2081] J Immunol. 1989;143:984–8. - PubMed
-
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

