A biomimetic peptide fluorosurfactant polymer for endothelialization of ePTFE with limited platelet adhesion
- PMID: 17507089
- PMCID: PMC2034336
- DOI: 10.1016/j.biomaterials.2007.04.026
A biomimetic peptide fluorosurfactant polymer for endothelialization of ePTFE with limited platelet adhesion
Abstract
Endothelialization of expanded polytetrafluoroethylene (ePTFE) has the potential to improve long-term patency for small-diameter vascular grafts. Successful endothelialization requires ePTFE surface modification to permit cell attachment to this otherwise non-adhesive substrate. We report here on a peptide fluorosurfactant polymer (FSP) biomimetic construct that promotes endothelial cell (EC)-selective attachment, growth, shear stability, and function on ePTFE. The peptide FSP consists of a flexible poly(vinyl amine) backbone with EC-selective peptide ligands for specific cell adhesion and pendant fluorocarbon branches for stable anchorage to underlying ePTFE. The EC-selective peptide (primary sequence: Cys-Arg-Arg-Glu-Thr-Ala-Trp-Ala-Cys, CRRETAWAC) has demonstrated high binding affinity for the alpha(5)beta(1) integrin found on ECs. Here, we demonstrate low affinity of CRRETAWAC for platelets and platelet integrins, thus providing it with EC-selectivity. This EC-selectivity could potentially facilitate rapid in vivo endothelialization and healing without thrombosis for small-diameter ePTFE vascular grafts.
Figures
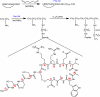

 ) or CRRETAWAC (
) or CRRETAWAC ( ) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for αIIbβ3 integrin. B) Inhibition of platelet aggregation by GRGDSP (
) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for αIIbβ3 integrin. B) Inhibition of platelet aggregation by GRGDSP ( ) or CRRETAWAC (
) or CRRETAWAC ( ) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for platelet receptors involved in aggregation.
) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for platelet receptors involved in aggregation.
 ) after exposure to static aqueous conditions for 4 and 20 weeks. Bars represent standard deviation for 3 measurements for each data point.
) after exposure to static aqueous conditions for 4 and 20 weeks. Bars represent standard deviation for 3 measurements for each data point.


 ), and RGD FSP (
), and RGD FSP ( ). ECs remained stably adherent to CRRETAWAC FSP after 4 h of 47.8 dynes/cm2 applied shear stress. * Significant difference in cell population (p<0.05) compared with 9.6 dynes/cm2 shear stress for same surface. B) Alignment of ECs on FN, CRRETAWAC FSP, and RGD FSP after 4 h of applied shear stress. ECs reoriented and aligned with shear upon application of minimal shear stress for all surfaces. * Significantly different (p<0.05) % of aligned cells compared with 9.6 and 38.2 dynes/cm2 shear stress for the same surface type. Bars represent standard deviation for 4 replicates per data point. C) Actin cytoskeleton staining for ECs on FN displaying alignment with shear after 4 h at 28.7 dynes/cm2. Scale bars for images are 100 μm. Arrows indicates direction of applied shear stress. D) Actin cytoskeleton staining for ECs on CRRETAWAC FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2. E) Actin cytoskeleton staining for ECs on RGD FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2.
). ECs remained stably adherent to CRRETAWAC FSP after 4 h of 47.8 dynes/cm2 applied shear stress. * Significant difference in cell population (p<0.05) compared with 9.6 dynes/cm2 shear stress for same surface. B) Alignment of ECs on FN, CRRETAWAC FSP, and RGD FSP after 4 h of applied shear stress. ECs reoriented and aligned with shear upon application of minimal shear stress for all surfaces. * Significantly different (p<0.05) % of aligned cells compared with 9.6 and 38.2 dynes/cm2 shear stress for the same surface type. Bars represent standard deviation for 4 replicates per data point. C) Actin cytoskeleton staining for ECs on FN displaying alignment with shear after 4 h at 28.7 dynes/cm2. Scale bars for images are 100 μm. Arrows indicates direction of applied shear stress. D) Actin cytoskeleton staining for ECs on CRRETAWAC FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2. E) Actin cytoskeleton staining for ECs on RGD FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2.

References
-
- Faries PL, Logerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000 Dec;32(6):1080–1090. - PubMed
-
- Sayers RD, Raptis S, Berce M, Miller JH. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg. 1998 Jul;85(7):934–938. - PubMed
-
- Laube HR, Duwe J, Rutsch W, Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg. 2000 Jul;120(1):134–141. - PubMed
-
- Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Froschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001 May;71(5 Suppl):S327–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

