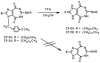
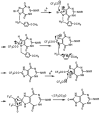
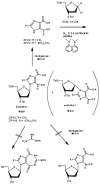
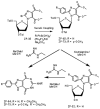
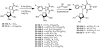Chemical and biological effects of substitution of the 2-position of ring-expanded ('fat') nucleosides containing the imidazo[4,5-e][1,3]diazepine-4,8-dione ring system: the role of electronic and steric factors on glycosidic bond stability and anti-HCV activity
- PMID: 17507230
- PMCID: PMC2754285
- DOI: 10.1016/j.bmc.2007.04.043
Chemical and biological effects of substitution of the 2-position of ring-expanded ('fat') nucleosides containing the imidazo[4,5-e][1,3]diazepine-4,8-dione ring system: the role of electronic and steric factors on glycosidic bond stability and anti-HCV activity
Abstract
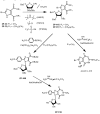
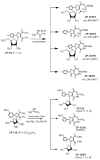
The attempted removal of the aralkyl group of 2-bromo-1-p-methoxybenzyl-6-octylimidazo[4,5-e][1,3]diazepine (ZP-33) with trifluoroacetic acid resulted in replacement of the bromo group with a carbonyl at position-2 in addition to the desired deprotection at the 1-position. 2'-Deoxynucleosides of 2-bromo-substituted-imidazole-4,5-diesters (ZP-35 and ZP-103) were synthesized by direct glycosylation of the corresponding heterocycles. The attempted ring-closure of the above nucleosides resulted in deglycosylation to form the starting heterocycles. The 2-phenyl derivatives of the above nucleosides (ZP-45 and ZP-73) were successfully prepared by Suzuki coupling with the appropriate phenylboronic acids, but the attempted ring-closure with guanidines to form the desired 5,7-fused ring-expanded nucleosides (RENs) resulted once again in the formation of the corresponding heterocyclic aglycons (ZP-64 and ZP-75). The first successful 2-substituted REN (ZP-110) was synthesized by replacing the 2-deoxyribose sugar moiety with a ribosyl group and replacing the bromo group with a p-methoxyphenyl substituent at the 2-position. A number of imidazole riboside diester precursors containing a substituted phenyl group at the 2-position were synthesized in order to prepare analogues of ZP-110. The substituents on the phenyl ring included a variety of electron-donating or electron-withdrawing groups operating through inductive and/or resonance effects. However, the final ring-closure of the diesters with guanidines in order to prepare RENs was successful only in a limited number of cases, including the ones containing a p-fluorophenyl (ZP-121), a m-methoxyphenyl (ZP-122), or an unsubstituted phenyl (NZ-53) at the 2-position. Deglycosylation and incomplete ring-closure of the intermediates were the major problems encountered with most other cases. The stability of glycosidic bonds was found to be dependent on several factors including, but not limited to, the electron-donating inductive effect of the 2-phenyl substituents and the temperature of the reaction medium. The three target RENs ZP-110, ZP-121, and ZP-122 were screened for in vitro anti-HCV activity, employing an HCV RNA replicon assay. While ZP-121 was inactive, the other two compounds showed only weak anti-HCV activity.
Figures
Similar articles
-
in vitro inhibition of the measles virus by novel ring-expanded ('fat') nucleoside analogues containing the imidazo[4,5-e]diazepine ring system.Bioorg Med Chem Lett. 2002 Dec 2;12(23):3391-4. doi: 10.1016/s0960-894x(02)00762-x. Bioorg Med Chem Lett. 2002. PMID: 12419368
-
Dual inhibition of HCV and HIV by ring-expanded nucleosides containing the 5:7-fused imidazo[4,5-e][1,3]diazepine ring system. In vitro results and implications.Bioorg Med Chem Lett. 2014 Feb 15;24(4):1154-7. doi: 10.1016/j.bmcl.2013.12.121. Epub 2014 Jan 8. Bioorg Med Chem Lett. 2014. PMID: 24461293 Free PMC article.
-
Synthesis and in vitro anti-hepatitis B and C virus activities of ring-expanded ('fat') nucleobase analogues containing the imidazo[4,5-e][1,3]diazepine-4,8-dione ring system.Bioorg Med Chem Lett. 2005 Dec 15;15(24):5397-401. doi: 10.1016/j.bmcl.2005.09.015. Epub 2005 Oct 5. Bioorg Med Chem Lett. 2005. PMID: 16213713
-
Ring-expanded ("Fat") nucleosides as broad-spectrum anticancer and antiviral agents.Curr Top Med Chem. 2002 Oct;2(10):1093-109. doi: 10.2174/1568026023393147. Curr Top Med Chem. 2002. PMID: 12173969 Review.
-
Synthesis of C-arylnucleoside analogues.Molecules. 2015 Mar 18;20(3):4967-97. doi: 10.3390/molecules20034967. Molecules. 2015. PMID: 25793544 Free PMC article. Review.
Cited by
-
Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer.Sci Rep. 2015 Apr 28;5:9982. doi: 10.1038/srep09982. Sci Rep. 2015. PMID: 25918862 Free PMC article.
References
-
- Zhang N, Chen HM, Koch V, Schmitz H, Liao CL, Bretner M, Bhadti VS, Fattom AI, Naso RB, Hosmane RS, Borowski P. J Med Chem. 2003;46:4149–4164. - PubMed
-
- Zhang N, Chen HM, Koch V, Schmitz H, Minczuk M, Stepien P, Fattom AI, Naso RB, Kalicharran K, Borowski P, Hosmane RS. J Med Chem. 2003;46:4776–4789. - PubMed
-
- Diamond MS, Klein RS. Trends Microbiol. 2006;14:287–289. - PubMed
-
- Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. Ann Neurol. 2006;60:286–300. - PubMed
-
- Hayes EB, Gubler DJ. Annu Rev Med. 2006;57:181–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources