Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord
- PMID: 17507415
- PMCID: PMC2804982
- DOI: 10.1242/dev.002980
Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord
Abstract
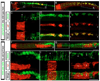
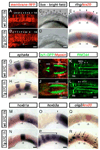
The spinal cord is a unique vertebrate feature that originates, together with the hindbrain, from the caudal neural plate. Whereas the hindbrain subdivides into rhombomeres, the spinal cord remains unsegmented. We have identified Cdx transcription factors as key determinants of the spinal cord region in zebrafish. Loss of Cdx1a and Cdx4 functions causes posterior expansion of the hindbrain at the expense of the unsegmented spinal cord. By contrast, cdx4 overexpression in the hindbrain impairs rhombomere segmentation and patterning and induces the expression of spinal cord-specific genes. Using cell transplantation, we demonstrate that Cdx factors function directly within the neural ectoderm to specify spinal cord. Overexpression of 5' Hox genes fails to rescue hindbrain and spinal cord defects associated with cdx1a/cdx4 loss-of-function, suggesting a Hox-independent mechanism of spinal cord specification. In the absence of Cdx function, the caudal neural plate retains hindbrain characteristics and remains responsive to surrounding signals, particularly retinoic acid, in a manner similar to the native hindbrain. We propose that by preventing the posterior-most region of the neural plate from following a hindbrain developmental program, Cdx factors help determine the size of the prospective hindbrain and spinal cord territories.
Figures



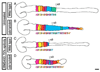

Similar articles
-
Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue.Development. 2006 Dec;133(23):4709-19. doi: 10.1242/dev.02660. Epub 2006 Nov 1. Development. 2006. PMID: 17079270
-
CDX4 and retinoic acid interact to position the hindbrain-spinal cord transition.Dev Biol. 2016 Feb 15;410(2):178-189. doi: 10.1016/j.ydbio.2015.12.025. Epub 2016 Jan 6. Dev Biol. 2016. PMID: 26773000 Free PMC article.
-
Retinoic acid regulates size, pattern and alignment of tissues at the head-trunk transition.Development. 2014 Nov;141(22):4375-84. doi: 10.1242/dev.109603. Development. 2014. PMID: 25371368
-
Hox genes and region-specific sensorimotor circuit formation in the hindbrain and spinal cord.Dev Dyn. 2013 Dec;242(12):1348-68. doi: 10.1002/dvdy.24055. Epub 2013 Nov 8. Dev Dyn. 2013. PMID: 23996673 Review.
-
Constructing the hindbrain: insights from the zebrafish.Dev Dyn. 2002 May;224(1):1-17. doi: 10.1002/dvdy.10086. Dev Dyn. 2002. PMID: 11984869 Review.
Cited by
-
Anterior-posterior patterning and segmentation of the vertebrate head.Integr Comp Biol. 2008 Nov;48(5):658-67. doi: 10.1093/icb/icn081. Epub 2008 Aug 5. Integr Comp Biol. 2008. PMID: 21669823 Free PMC article.
-
Revisiting the segmental organization of the human spinal cord.J Anat. 2016 Sep;229(3):384-93. doi: 10.1111/joa.12493. Epub 2016 May 12. J Anat. 2016. PMID: 27173936 Free PMC article.
-
Zebrafish Tbx16 regulates intermediate mesoderm cell fate by attenuating Fgf activity.Dev Biol. 2013 Nov 1;383(1):75-89. doi: 10.1016/j.ydbio.2013.08.018. Epub 2013 Sep 2. Dev Biol. 2013. PMID: 24008197 Free PMC article.
-
A forward chemical screen in zebrafish identifies a retinoic acid derivative with receptor specificity.PLoS One. 2010 Apr 2;5(4):e10004. doi: 10.1371/journal.pone.0010004. PLoS One. 2010. PMID: 20368991 Free PMC article.
-
Enhanced production of mesencephalic dopaminergic neurons from lineage-restricted human undifferentiated stem cells.Nat Commun. 2023 Dec 5;14(1):7871. doi: 10.1038/s41467-023-43471-0. Nat Commun. 2023. PMID: 38052784 Free PMC article.
References
-
- Begemann G, Meyer A. Hindbrain patterning revisited: timing and effects of retinoic acid signalling. BioEssays. 2001;23:981–986. - PubMed
-
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. - PubMed
-
- Begemann G, Marx M, Mebus K, Meyer A, Bastmeyer M. Beyond the neckless phenotype: influence of reduced retinoic acid signaling on motor neuron development in the zebrafish hindbrain. Dev. Biol. 2004;271:119–129. - PubMed
-
- Bone Q. The central nervous system in Amphioxus. J. Comp. Neurol. 1960;115:27–64.
-
- Borday C, Wrobel L, Fortin G, Champagnat J, Thaeron-Antono C, Thoby-Brisson M. Developmental gene control of brainstem function: views from the embryo. Prog. Biophys. Mol. Biol. 2004;84:89–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

