Evidence for cooperative transforming activity of the human pituitary tumor transforming gene and human T-cell leukemia virus type 1 Tax
- PMID: 17507465
- PMCID: PMC1951308
- DOI: 10.1128/JVI.00555-07
Evidence for cooperative transforming activity of the human pituitary tumor transforming gene and human T-cell leukemia virus type 1 Tax
Abstract
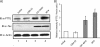
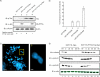
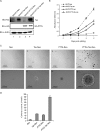
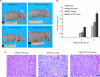
Aneuploidy is frequent in cancers. Recently it was found that pituitary tumor transforming gene (PTTG; also called Pds1p or securin) is overexpressed in many different tumors. Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus that primarily infects CD4+ T lymphocytes and causes adult T-cell leukemia. Here, we report that overexpression of human PTTG cooperated with the HTLV-I Tax oncoprotein in cellular transformation. Coexpression of Tax and PTTG enhanced chromosomal instability and neoplastic changes to levels greater than overexpression of either factor singularly. Cells that overexpressed both PTTG and Tax induced tumors more robustly in nude mice than cells that expressed either PTTG alone or Tax alone.
Figures

References
-
- Akagi, T., H. Ono, N. Tsuchida, and K. Shimotohno. 1997. Aberrant expression and function of p53 in T-cells immortalized by HTLV-I Tax1. FEBS Lett. 406:263-266. - PubMed
-
- Anderson, M. D., J. Ye, L. Xie, and P. L. Green. 2004. Transformation studies with a human T-cell leukemia virus type 1 molecular clone. J. Virol. Methods 116:195-202. - PubMed
-
- Ariumi, Y., A. Kaida, J. Y. Lin, M. Hirota, O. Masui, S. Yamaoka, Y. Taya, and K. Shimotohno. 2000. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 19:1491-1499. - PubMed
-
- Bernal, J. A., R. Luna, A. Espina, I. Lazaro, F. Ramos-Morales, F. Romero, C. Arias, A. Silva, M. Tortolero, and J. A. Pintor-Toro. 2002. Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis. Nat. Genet. 32:306-311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

