Surface-exposed adeno-associated virus Vp1-NLS capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions
- PMID: 17507473
- PMCID: PMC1951316
- DOI: 10.1128/JVI.00580-07
Surface-exposed adeno-associated virus Vp1-NLS capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions
Abstract
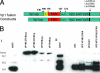
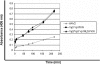
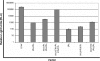
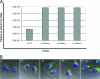
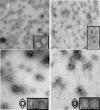
Over the past 2 decades, significant effort has been dedicated to the development of adeno-associated virus (AAV) as a vector for human gene therapy. However, understanding of the virus with respect to the functional domains of the capsid remains incomplete. In this study, the goal was to further examine the role of the unique Vp1 N terminus, the N terminus plus the recently identified nuclear localization signal (NLS) (J. C. Grieger, S. Snowdy, and R. J. Samulski, J. Virol 80:5199-5210, 2006), and the virion pore at the fivefold axis in infection. We generated two Vp1 fusion proteins (Vp1 and Vp1NLS) linked to the 8-kDa chemokine domain of rat fractalkine (FKN) for the purpose of surface exposure upon assembly of the virion, as previously described (K. H. Warrington, Jr., O. S. Gorbatyuk, J. K. Harrison, S. R. Opie, S. Zolotukhin, and N. Muzyczka, J. Virol 78:6595-6609, 2004). The unique Vp1 N termini were found to be exposed on the surfaces of these capsids and maintained their phospholipase A2 (PLA2) activity, as determined by native dot blot Western and PLA2 assays, respectively. Incorporation of the fusions into AAV type 2 capsids lacking a wild-type Vp1, i.e., Vp2/Vp3 and Vp3 capsid only, increased infectivity by 3- to 5-fold (Vp1FKN) and 10- to 100-fold (Vp1NLSFKN), respectively. However, the surface-exposed fusions did not restore infectivity to AAV virions containing mutations at a conserved leucine (Leu336Ala, Leu336Cys, or Leu336Trp) located at the base of the fivefold pore. EM analyses suggest that Leu336 may play a role in global structural changes to the virion directly impacting downstream conformational changes essential for infectivity and not only have local effects within the pore, as previously suggested.
Figures



References
-
- Agbandje, M., S. Kajigaya, R. McKenna, N. S. Young, and M. G. Rossmann. 1994. The structure of human parvovirus B19 at 8 Å resolution. Virology 203:106-115. - PubMed
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Agbandje-McKenna, M., A. L. Llamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus, minute virus of mice. Structure 6:1369-1381. - PubMed
-
- Atchison, R. W., B. C. Casto, and W. M. Hammon. 1965. Adenovirus-associated defective virus particles. Science 149:754-756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

