Varicella-Zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response
- PMID: 17507475
- PMCID: PMC1951283
- DOI: 10.1128/JVI.00325-07
Varicella-Zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response
Abstract
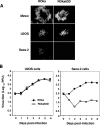
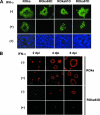
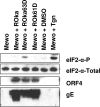
Varicella-zoster virus (VZV) open reading frame 63 (ORF63) is the most abundant transcript expressed during latency in human sensory ganglia. VZV with ORF63 deleted is impaired for replication in melanoma cells and fibroblasts and for latency in rodents. We found that replication of the ORF63 deletion mutant is fully complemented in U2OS cells, which have been shown to complement the growth of herpes simplex virus type 1 (HSV-1) ICP0 mutants. Since HSV-1 ICP0 mutants are hypersensitive to alpha interferon (IFN-alpha), we examined the effect of IFN-alpha on VZV replication. Replication of the ORF63 mutant in melanoma cells was severely inhibited in the presence of IFN-alpha, in contrast to other VZV mutants that were similarly impaired for replication or to parental virus. The VZV ORF63 mutant was not hypersensitive to IFN-gamma. IFN-alpha inhibited viral-gene expression in cells infected with the ORF63 mutant at a posttranscriptional level. Since IFN-alpha stimulates gene products that can phosphorylate the alpha subunit of eukaryotic initiation factor 2 (eIF-2alpha) and inhibit translation, we determined whether cells infected with the ORF63 mutant had increased phosphorylation of eIF-2alpha compared with cells infected with parental virus. While phosphorylated eIF-2alpha was undetectable in uninfected cells or cells infected with parental virus, it was present in cells infected with the ORF63 mutant. Conversely, expression of IE63 (encoded by ORF63) in the absence of other viral proteins inhibited phosphorylation of eIF-2alpha. Since IFN-alpha has been shown to limit VZV replication in human skin xenografts, the ability of VZV IE63 to block the effects of the cytokine may play a critical role in VZV pathogenesis.
Figures




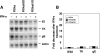

References
-
- Arvin, A. M., J. H. Kushner, S. Feldman, R. L. Baehner, D. Hammond, and T. C. Merigan. 1982. Human leukocyte interferon for the treatment of varicella in children with cancer. N. Engl. J. Med. 306:761-765. - PubMed
-
- Baiker, A., C. Bagowski, H. Ito, M. Sommer, L. Zerboni, K. Fabel, J. Hay, W. Ruyechan, and A. M. Arvin. 2004. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 78:1181-1194. - PMC - PubMed
-
- Balachandra, K., D. Thawaranantha, P. I. Ayuthaya, J. Bhumisawasdi, K. Shiraki, and K. Yamanishi. 1994. Effects of human alpha, beta and gamma interferons on varicella zoster virus in vitro. Southeast Asian J. Trop. Med. Public Health 25:252-257. - PubMed
-
- Bontems, S., E. Di Valentin, L. Baudoux, B. Rentier, C. Sadzot-Delvaux, and J. Piette. 2002. Phosphorylation of varicella-zoster virus IE63 protein by casein kinases influences its cellular localization and gene regulation activity. J. Biol. Chem. 277:21050-21060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

