Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence
- PMID: 17507481
- PMCID: PMC1933358
- DOI: 10.1128/JVI.02839-06
Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence
Abstract
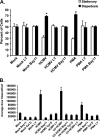
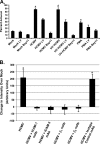
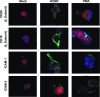
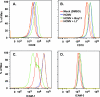
Infected peripheral blood monocytes are proposed to play a key role in the hematogenous dissemination of human cytomegalovirus (HCMV) to tissues, a critical step in the establishment of HCMV persistence and the development of HCMV-associated diseases. We recently provided evidence for a unique strategy involved in viral dissemination: HCMV infection of primary human monocytes promotes their transendothelial migration and differentiation into proinflammatory macrophages permissive for the replication of the original input virus. To decipher the mechanism of hematogenous spread, we focused on the viral dysregulation of early cellular processes involved in transendothelial migration. Here, we present evidence that both phosphatidylinositol 3-kinase [PI(3)K] and NF-kappaB activities were crucial for the HCMV induction of monocyte motility and firm adhesion to endothelial cells. We found that the beta(1) integrins, the beta(2) integrins, intracellular adhesion molecule 1 (ICAM-1), and ICAM-3 were upregulated following HCMV infection and that they played a key role in the firm adhesion of infected monocytes to the endothelium. The viral regulation of adhesion molecule expression is complex, with PI(3)K and NF-kappaB affecting the expression of each adhesion molecule at different stages of the expression cascade. Our data demonstrate key roles for PI(3)K and NF-kappaB signaling in the HCMV-induced cellular changes in monocytes and identify the biological rationale for the activation of these pathways in infected monocytes, which together suggest a mechanism for how HCMV promotes viral spread to and persistence within host organs.
Figures



References
-
- Albrecht-Buehler, G. 1977. The phagokinetic tracks of 3T3 cells. Cell 11:395-404. - PubMed
-
- Barja-Fidalgo, C., A. L. Coelho, R. Saldanha-Gama, E. Helal-Neto, A. Mariano-Oliveira, and M. S. Freitas. 2005. Disintegrins: integrin selective ligands which activate integrin-coupled signaling and modulate leukocyte functions. Braz. J. Med. Biol. Res. 38:1513-1520. - PubMed
-
- Bentz, G. L., M. Jarquin-Pardo, G. Chan, M. S. Smith, C. Sinzger, and A. D. Yurochko. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naïve monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 80:11539-11555. - PMC - PubMed
-
- Boehme, K. W., M. Guerrero, and T. Compton. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094-7102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

