Productive human immunodeficiency virus type 1 assembly takes place at the plasma membrane
- PMID: 17507489
- PMCID: PMC1933344
- DOI: 10.1128/JVI.00308-07
Productive human immunodeficiency virus type 1 assembly takes place at the plasma membrane
Abstract
Gag proteins are necessary and sufficient to direct human immunodeficiency virus type 1 (HIV-1) particle assembly and budding. Recent evidence suggests that Gag targeting to late endosomal/multivesicular body (LE/MVB) compartments occurs prior to viral particle budding at the plasma membrane (PM). However, the route that Gag follows before reaching its steady-state destinations still remains a subject of debate. Using a subcellular fractionation method that separates PM from LE/MVB combined with pulse-chase labeling, we analyzed Gag trafficking in HIV-1-producing HEK 293T cells. Our results reveal that the majority of newly synthesized Gag is primarily targeted to the PM. While PM-targeted Gag was efficiently released, a significant fraction of the remaining cell surface-associated Gag was found to be subsequently internalized to LE/MVB, where it accumulated, thus accounting for the majority of LE/MVB-associated Gag. Importantly, this accumulation of Gag in LE/MVB was found to be cholesterol dependent since it was sensitive to the sterol-binding drugs filipin and methyl-beta-cyclodextrin. These results point towards the PM as being the primary site of productive HIV-1 assembly in cells that also support Gag accumulation in intracellular compartments.
Figures
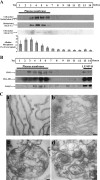



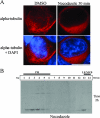
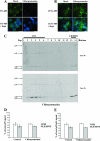

References
-
- Abedinpour, P., and B. Jergil. 2003. Isolation of a caveolae-enriched fraction from rat lung by affinity partitioning and sucrose gradient centrifugation. Anal. Biochem. 313:1-8. - PubMed
-
- Beriault, V., J. F. Clement, K. Levesque, C. Lebel, X. Yong, B. Chabot, E. A. Cohen, A. W. Cochrane, W. F. Rigby, and A. J. Mouland. 2004. A late role for the association of hnRNP A2 with the HIV-1 hnRNP A2 response elements in genomic RNA, Gag, and Vpr localization. J. Biol. Chem. 279:44141-44153. - PubMed
-
- Bieniasz, P. D. 2006. Late budding domains and host proteins in enveloped virus release. Virology 344:55-63. - PubMed
-
- Bomsel, M., R. Parton, S. A. Kuznetsov, T. A. Schroer, and J. Gruenberg. 1990. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62:719-731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

