DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge
- PMID: 17507492
- PMCID: PMC1933361
- DOI: 10.1128/JVI.00633-07
DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge
Abstract
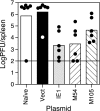
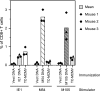
Human cytomegalovirus (HCMV) establishes a lifelong infection with the potential for reinfection or viral transmission even in the presence of strong and diverse CD8 T-lymphocyte responses. This suggests that the CMVs skew the host T-cell response in order to favor viral persistence. In this study, we hypothesized that the essential, nonstructural proteins that are highly conserved among the CMVs may represent a novel class of T-cell targets for vaccine-mediated protection due to their requirements for expression and sequence stability, but that the observed subdominance of these antigens in the CMV-infected host results from the virus limiting the T-cell responses to otherwise-protective specificities. We found that DNA immunization of mice with the murine CMV (MCMV) homologs of HCMV DNA polymerase (M54) or helicase (M105) was protective against virus replication in the spleen following systemic challenge, with the protection level elicited by the M54 DNA being comparable to that of DNA expressing the immunodominant IE1 (pp89). Intracellular gamma interferon staining of CD8 T cells from mice immunized with either the M54 or M105 DNAs showed strong primary responses that recalled rapidly after viral challenge. M54- and M105-specific CD8 T cells were detected after the primary MCMV infection, but their levels were not consistently above the background level. The conserved, essential proteins of the CMVs thus represent a novel class of CD8 T-cell targets that may contribute to a successful HCMV vaccine strategy.
Figures
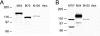
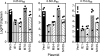

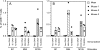
References
-
- Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323-1326. - PubMed
-
- Brune, W., H. Hengel, and U. H. Koszinowski. 1999. A mouse model for cytomegalovirus infection, p. 19.17.11-19.17.13. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, vol. 4. John Wiley & Sons, Inc., New York, NY. - PubMed
-
- Cranmer, L. D., C. L. Clark, C. S. Morello, H. E. Farrell, W. D. Rawlinson, and D. H. Spector. 1996. Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J. Virol. 70:7929-7939. - PMC - PubMed
-
- Elliott, R., C. Clark, D. Jaquish, and D. H. Spector. 1991. Transcription analysis and sequence of the putative murine cytomegalovirus DNA polymerase gene. Virology 185:169-186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

