Genetic single-cell mosaic analysis implicates ephrinB2 reverse signaling in projections from the posterior tectum to the hindbrain in zebrafish
- PMID: 17507550
- PMCID: PMC6672335
- DOI: 10.1523/JNEUROSCI.0883-07.2007
Genetic single-cell mosaic analysis implicates ephrinB2 reverse signaling in projections from the posterior tectum to the hindbrain in zebrafish
Abstract
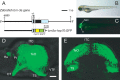
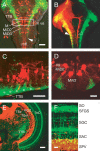
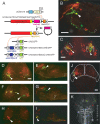
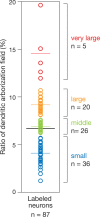
The optic tectum is a visual center in vertebrates. It receives topographically ordered visual inputs from the retina in the superficial layers and then sends motor outputs from the deeper layers to the premotor reticulospinal system in the hindbrain. Although the topographic patterns of the retinotectal projection are well known, it is not yet well understood how tectal efferents in the tectobulbar tract project to the hindbrain. The retinotectal and the tectobulbar projections were visualized in a zebrafish stable transgenic line Tg(brn3a-hsp70:GFP). Using a single-neuron labeling system in combination with the cre/loxP and Gal4/UAS systems, we showed that the tectal neurons that projected to rhombomeres 2 and 6 were distributed with distinctive patterns along the anterior-posterior axis. Furthermore, we found that ephrinB2a was critically involved in increasing the probability of neurons projecting to rhombomere 2 through a reverse signaling mechanism. These results may provide a neuroanatomical and molecular basis for the motor command map in the tectum.
Figures



Similar articles
-
Neuron types in the zebrafish optic tectum labeled by an id2b transgene.J Comp Neurol. 2020 May;528(7):1173-1188. doi: 10.1002/cne.24815. Epub 2019 Nov 26. J Comp Neurol. 2020. PMID: 31725916
-
EphrinB2a in the zebrafish retinotectal system.J Neurobiol. 2004 Apr;59(1):57-65. doi: 10.1002/neu.10340. J Neurobiol. 2004. PMID: 15007827
-
Meis1 specifies positional information in the retina and tectum to organize the zebrafish visual system.Neural Dev. 2010 Sep 1;5:22. doi: 10.1186/1749-8104-5-22. Neural Dev. 2010. PMID: 20809932 Free PMC article.
-
Roles of periventricular neurons in retinotectal transmission in the optic tectum.Prog Neurobiol. 2006 Jun;79(2):112-21. doi: 10.1016/j.pneurobio.2006.06.002. Epub 2006 Aug 9. Prog Neurobiol. 2006. PMID: 16901616 Review.
-
The molecular basis of retinotectal topography.Bioessays. 1994 Jan;16(1):1-11. doi: 10.1002/bies.950160102. Bioessays. 1994. PMID: 7908192 Review.
Cited by
-
Social Status-Dependent Shift in Neural Circuit Activation Affects Decision Making.J Neurosci. 2017 Feb 22;37(8):2137-2148. doi: 10.1523/JNEUROSCI.1548-16.2017. Epub 2017 Jan 16. J Neurosci. 2017. PMID: 28093472 Free PMC article.
-
Gal80 intersectional regulation of cell-type specific expression in vertebrates.Dev Dyn. 2011 Oct;240(10):2324-34. doi: 10.1002/dvdy.22734. Epub 2011 Sep 8. Dev Dyn. 2011. PMID: 21905164 Free PMC article.
-
Neuregulin 1 Type II-ErbB Signaling Promotes Cell Divisions Generating Neurons from Neural Progenitor Cells in the Developing Zebrafish Brain.PLoS One. 2015 May 22;10(5):e0127360. doi: 10.1371/journal.pone.0127360. eCollection 2015. PLoS One. 2015. PMID: 26001123 Free PMC article.
-
Neuronal connectivity between habenular glutamate-kisspeptin1 co-expressing neurons and the raphe 5-HT system.J Neurochem. 2015 Nov;135(4):814-29. doi: 10.1111/jnc.13273. Epub 2015 Sep 10. J Neurochem. 2015. PMID: 26250886 Free PMC article.
-
New Insights into the Identity of the DFNA58 Gene.Genes (Basel). 2022 Dec 2;13(12):2274. doi: 10.3390/genes13122274. Genes (Basel). 2022. PMID: 36553541 Free PMC article.
References
-
- Al-Akel AS, Guthrie DM, Banks JR. Motor responses to localized electrical stimulation of the tectum in the freshwater perch (Perca fluviatilis) Neuroscience. 1986;19:1381–1391. - PubMed
-
- Bovenkamp DE, Greer P. Novel Eph-family receptor tyrosine kinase is widely expressed in the developing zebrafish nervous system. Dev Dyn. 1997;209:166–181. - PubMed
-
- Chan J, Mably JD, Serluca FC, Chen JN, Goldstein NB, Thomas MC, Cleary JA, Brennan C, Fishman MC, Roberts TM. Morphogenesis of prechordal plate and notochord requires intact Eph/ephrin B signaling. Dev Biol. 2001;234:470–482. - PubMed
-
- Cooke J, Moens C, Roth L, Durbin L, Shiomi K, Brennan C, Kimmel C, Wilson S, Holder N. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development. 2001;128:571–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
