Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex
- PMID: 17507562
- PMCID: PMC6672348
- DOI: 10.1523/JNEUROSCI.5425-06.2007
Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex
Abstract
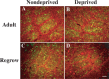
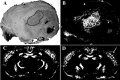
An important role for the neural extracellular matrix in modulating cortical activity-dependent synaptic plasticity has been established by a number of recent studies. However, identification of the critical molecular components of the neural matrix that mediate these processes is far from complete. Of particular interest is the perineuronal net (PN), an extracellular matrix component found surrounding the cell body and proximal neurites of a subset of neurons. Because of the apposition of the PN to synapses and expression of this structure coincident with the close of the critical period, it has been hypothesized that nets could play uniquely important roles in synapse stabilization and maturation. Interestingly, previous work has also shown that expression of PNs is dependent on appropriate sensory stimulation in the visual system. Here, we investigated whether PNs in the mouse barrel cortex are expressed in an activity-dependent manner by manipulating sensory input through whisker trimming. Importantly, this manipulation did not lead to a global loss of PNs but instead led to a specific decrease in PNs, detected with the antibody Cat-315, in layer IV of the barrel cortex. In addition, we identified a key activity-regulated component of PNs is the proteoglycan aggrecan. We also demonstrate that these Cat-315-positive neurons virtually all also express parvalbumin. Together, these data are in support of an important role for aggrecan in the activity-dependent formation of PNs on parvalbumin-expressing cells and suggest a role for expression of these nets in regulating the close of the critical period.
Figures





Similar articles
-
Sensory experience-dependent formation of perineuronal nets and expression of Cat-315 immunoreactive components in the mouse somatosensory cortex.Neuroscience. 2017 Jul 4;355:161-174. doi: 10.1016/j.neuroscience.2017.04.041. Epub 2017 May 8. Neuroscience. 2017. PMID: 28495333
-
Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex.Matrix Biol. 2013 Aug 8;32(6):352-63. doi: 10.1016/j.matbio.2013.04.001. Epub 2013 Apr 15. Matrix Biol. 2013. PMID: 23597636
-
Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex.Eur J Neurosci. 2009 Dec 3;30(11):2053-63. doi: 10.1111/j.1460-9568.2009.06996.x. Epub 2009 Nov 25. Eur J Neurosci. 2009. PMID: 20128844
-
Aggrecan: Beyond cartilage and into the brain.Int J Biochem Cell Biol. 2012 May;44(5):690-3. doi: 10.1016/j.biocel.2012.01.010. Epub 2012 Jan 25. Int J Biochem Cell Biol. 2012. PMID: 22297263 Review.
-
Human brain plasticity: evidence from sensory deprivation and altered language experience.Prog Brain Res. 2002;138:177-88. doi: 10.1016/S0079-6123(02)38078-6. Prog Brain Res. 2002. PMID: 12432770 Review.
Cited by
-
Chemistry and Function of Glycosaminoglycans in the Nervous System.Adv Neurobiol. 2023;29:117-162. doi: 10.1007/978-3-031-12390-0_5. Adv Neurobiol. 2023. PMID: 36255674 Review.
-
A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders.Front Cell Neurosci. 2015 Jul 29;9:280. doi: 10.3389/fncel.2015.00280. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26283917 Free PMC article. Review.
-
Releasing Addiction Memories Trapped in Perineuronal Nets.Trends Genet. 2018 Mar;34(3):197-208. doi: 10.1016/j.tig.2017.12.004. Epub 2017 Dec 27. Trends Genet. 2018. PMID: 29289347 Free PMC article. Review.
-
A comprehensive atlas of Aggrecan, Versican, Neurocan and Phosphacan expression across time in wildtype retina and in retinal degeneration.Sci Rep. 2022 May 4;12(1):7282. doi: 10.1038/s41598-022-11204-w. Sci Rep. 2022. PMID: 35508614 Free PMC article.
-
Glial regulation of critical period plasticity.Front Cell Neurosci. 2023 Nov 16;17:1247335. doi: 10.3389/fncel.2023.1247335. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38034592 Free PMC article. Review.
References
-
- Brauer K, Hartig W, Bigl V, Bruckner G. Distribution of parvalbumin-containing neurons and lectin-binding perineuronal nets in the rat basal forebrain. Brain Res. 1993;631:167–170. - PubMed
-
- Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. - PubMed
-
- Bruckner G, Bringmann A, Koppe G, Hartig W, Brauer K. In vivo and in vitro labelling of perineuronal nets in rat brain. Brain Res. 1996;720:84–92. - PubMed
-
- Bruckner G, Bringmann A, Hartig W, Koppe G, Delpech B, Brauer K. Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp Brain Res. 1998;121:300–310. - PubMed
-
- Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
