Fast vesicle replenishment and rapid recovery from desensitization at a single synaptic release site
- PMID: 17507567
- PMCID: PMC6672343
- DOI: 10.1523/JNEUROSCI.1186-07.2007
Fast vesicle replenishment and rapid recovery from desensitization at a single synaptic release site
Abstract
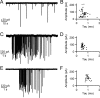
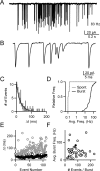
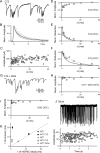
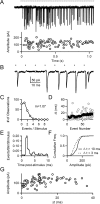
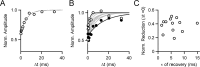
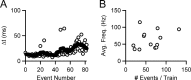
When the synaptic connection between two neurons consists of a small number of release sites, the ability to maintain transmission at high frequencies is limited by vesicle mobilization and by the response of postsynaptic receptors. These two properties were examined at single release sites between granule cells and stellate cells by triggering bursts of quantal events either with alpha-latrotoxin or with high-frequency trains of presynaptic activity. Bursts and evoked responses consisted of tens to hundreds of events with frequencies of up to hundreds per second. This indicates that single release sites can rapidly supply vesicles from a reserve pool to a release-ready pool. In addition, postsynaptic AMPA receptors recover from desensitization with a time constant of approximately 5 ms. Thus, even for synapses composed of a single release site, granule cells can effectively activate stellate cells during sustained high-frequency transmission because of rapid vesicle mobilization and fast recovery of AMPA receptors from desensitization.
Figures



Similar articles
-
Counting Vesicular Release Events Reveals Binomial Release Statistics at Single Glutamatergic Synapses.J Neurosci. 2016 Apr 6;36(14):4010-25. doi: 10.1523/JNEUROSCI.4352-15.2016. J Neurosci. 2016. PMID: 27053208 Free PMC article.
-
Two Ca(2+)-dependent steps controlling synaptic vesicle fusion and replenishment at the cerebellar basket cell terminal.Neuron. 2008 Feb 7;57(3):406-19. doi: 10.1016/j.neuron.2007.11.029. Neuron. 2008. PMID: 18255033
-
Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS.J Physiol. 2005 Oct 15;568(Pt 2):513-37. doi: 10.1113/jphysiol.2005.093468. Epub 2005 Aug 11. J Physiol. 2005. PMID: 16096340 Free PMC article.
-
Multiple vesicle recycling pathways in central synapses and their impact on neurotransmission.J Physiol. 2007 Dec 15;585(Pt 3):669-79. doi: 10.1113/jphysiol.2007.137745. Epub 2007 Aug 9. J Physiol. 2007. PMID: 17690145 Free PMC article. Review.
-
Sustained neurotransmitter release: new molecular clues.Eur J Neurosci. 1997 Dec;9(12):2503-11. doi: 10.1111/j.1460-9568.1997.tb01679.x. Eur J Neurosci. 1997. PMID: 9517455 Review.
Cited by
-
High frequency burst firing of granule cells ensures transmission at the parallel fiber to purkinje cell synapse at the cost of temporal coding.Front Neural Circuits. 2013 May 21;7:95. doi: 10.3389/fncir.2013.00095. eCollection 2013. Front Neural Circuits. 2013. PMID: 23734102 Free PMC article.
-
Synaptic Multivesicular Release in the Cerebellar Cortex: Its Mechanism and Role in Neural Encoding and Processing.Cerebellum. 2016 Apr;15(2):201-7. doi: 10.1007/s12311-015-0677-5. Cerebellum. 2016. PMID: 25971904
-
The ubiquitous nature of multivesicular release.Trends Neurosci. 2015 Jul;38(7):428-38. doi: 10.1016/j.tins.2015.05.008. Epub 2015 Jun 19. Trends Neurosci. 2015. PMID: 26100141 Free PMC article. Review.
-
What We Can Learn From Cumulative Numbers of Vesicular Release Events.Front Cell Neurosci. 2019 Jun 21;13:257. doi: 10.3389/fncel.2019.00257. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31293386 Free PMC article.
-
Activation of extrasynaptic NMDARs at individual parallel fiber-molecular layer interneuron synapses in cerebellum.J Neurosci. 2013 Oct 9;33(41):16323-33. doi: 10.1523/JNEUROSCI.1971-13.2013. J Neurosci. 2013. PMID: 24107963 Free PMC article.
References
-
- Arai A, Lynch G. AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res. 1998;799:235–242. - PubMed
-
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. - PubMed
-
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
