Learning-induced plasticity in auditory spatial representations revealed by electrical neuroimaging
- PMID: 17507569
- PMCID: PMC6672359
- DOI: 10.1523/JNEUROSCI.0764-07.2007
Learning-induced plasticity in auditory spatial representations revealed by electrical neuroimaging
Abstract
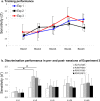
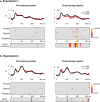
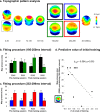
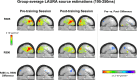
Auditory spatial representations are likely encoded at a population level within human auditory cortices. We investigated learning-induced plasticity of spatial discrimination in healthy subjects using auditory-evoked potentials (AEPs) and electrical neuroimaging analyses. Stimuli were 100 ms white-noise bursts lateralized with varying interaural time differences. In three experiments, plasticity was induced with 40 min of discrimination training. During training, accuracy significantly improved from near-chance levels to approximately 75%. Before and after training, AEPs were recorded to stimuli presented passively with a more medial sound lateralization outnumbering a more lateral one (7:1). In experiment 1, the same lateralizations were used for training and AEP sessions. Significant AEP modulations to the different lateralizations were evident only after training, indicative of a learning-induced mismatch negativity (MMN). More precisely, this MMN at 195-250 ms after stimulus onset followed from differences in the AEP topography to each stimulus position, indicative of changes in the underlying brain network. In experiment 2, mirror-symmetric locations were used for training and AEP sessions; no training-related AEP modulations or MMN were observed. In experiment 3, the discrimination of trained plus equidistant untrained separations was tested psychophysically before and 0, 6, 24, and 48 h after training. Learning-induced plasticity lasted <6 h, did not generalize to untrained lateralizations, and was not the simple result of strengthening the representation of the trained lateralizations. Thus, learning-induced plasticity of auditory spatial discrimination relies on spatial comparisons, rather than a spatial anchor or a general comparator. Furthermore, cortical auditory representations of space are dynamic and subject to rapid reorganization.
Figures

Similar articles
-
Auditory detection learning is accompanied by plasticity in the auditory evoked potential.Neurosci Lett. 2020 Mar 16;721:134781. doi: 10.1016/j.neulet.2020.134781. Epub 2020 Jan 28. Neurosci Lett. 2020. PMID: 32004657
-
Increased MMN amplitude following passive perceptual learning with LTP-like rapid stimulation.Neurosci Lett. 2018 Feb 14;666:28-31. doi: 10.1016/j.neulet.2017.12.035. Epub 2017 Dec 15. Neurosci Lett. 2018. PMID: 29253602
-
Plastic changes in the auditory cortex induced by intensive frequency discrimination training.Neuroreport. 2000 Mar 20;11(4):817-22. doi: 10.1097/00001756-200003200-00032. Neuroreport. 2000. PMID: 10757526
-
Learning-induced plasticity in human audition: objects, time, and space.Hear Res. 2011 Jan;271(1-2):88-102. doi: 10.1016/j.heares.2010.03.086. Epub 2010 Apr 27. Hear Res. 2011. PMID: 20430070 Review.
-
The Mismatch Negativity: An Indicator of Perception of Regularities in Music.Behav Neurol. 2015;2015:469508. doi: 10.1155/2015/469508. Epub 2015 Oct 4. Behav Neurol. 2015. PMID: 26504352 Free PMC article. Review.
Cited by
-
Cortical processing of speech in individuals with auditory neuropathy spectrum disorder.Eur Arch Otorhinolaryngol. 2018 Jun;275(6):1409-1418. doi: 10.1007/s00405-018-4966-8. Epub 2018 Apr 9. Eur Arch Otorhinolaryngol. 2018. PMID: 29633023
-
Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia.Neuropsychiatr Electrophysiol. 2017;3:2. doi: 10.1186/s40810-017-0024-9. Epub 2017 Jun 9. Neuropsychiatr Electrophysiol. 2017. PMID: 28845238 Free PMC article.
-
Age-related changes in visually evoked electrical brain activity.Hum Brain Mapp. 2012 May;33(5):1124-36. doi: 10.1002/hbm.21273. Epub 2011 Apr 29. Hum Brain Mapp. 2012. PMID: 21538705 Free PMC article. Clinical Trial.
-
Analyzing variability in neural responses to complex natural sounds in the awake songbird.J Neurophysiol. 2009 Jun;101(6):3147-57. doi: 10.1152/jn.90917.2008. Epub 2009 Apr 8. J Neurophysiol. 2009. PMID: 19357333 Free PMC article.
-
Early, low-level auditory-somatosensory multisensory interactions impact reaction time speed.Front Integr Neurosci. 2009 Mar 11;3:2. doi: 10.3389/neuro.07.002.2009. eCollection 2009. Front Integr Neurosci. 2009. PMID: 19404410 Free PMC article.
References
-
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. - PubMed
-
- Altman YA, Vaitulevich SF, Shestopalova LB. Changes in evoked potentials during the action of sound signals with different localizing characteristics. Neurosci Behav Physiol. 2004;34:139–146. - PubMed
-
- Atienza M, Cantero JL, Quian Quiroga R. Precise timing accounts for post-training sleep-dependent enhancements of the auditory mismatch negativity. NeuroImage. 2005;26:628–634. - PubMed
-
- Barbay S, Peden EK, Falchook G, Nudo RJ. Sensitivity of neurons in somatosensory cortex (S1) to cutaneous stimulation of the hindlimb immediately following a sciatic nerve crush. Somatosens Mot Res. 1999;16:103–114. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
