Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia
- PMID: 17507571
- PMCID: PMC6672350
- DOI: 10.1523/JNEUROSCI.1384-07.2007
Prolonged glial expression of Sox4 in the CNS leads to architectural cerebellar defects and ataxia
Abstract
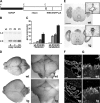
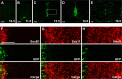
Sox proteins of group C are strongly expressed in the developing nervous system and have been associated with maturation of neurons and glia. Here, we overexpressed the group C protein Sox4 in transgenic mice under the control of the human GFAP promoter. Transgene expression was detected in radial glia and astrocytes throughout the CNS. The transgenic mice were ataxic and exhibited hydrocephaly as well as cerebellar malformations. In the cerebellum, fissures were not formed and neuronal layering was dramatically disturbed. Nevertheless, all neuronal cell types of the cerebellum were present as well as cells with characteristics of early radial glia, astrocytes, and oligodendrocytes. However, radial glia failed to migrate into the position normally taken by Bergmann glia and did not extend radial fibers toward the pial surface. The cerebellar malformations can therefore be explained by the absence of functional Bergmann glia. We conclude that Sox4 expression counteracts differentiation of radial glia and has to be downregulated before full maturation can occur.
Figures






References
-
- Baader SL, Bergmann M, Mertz KD, Fox PA, Gerdes J, Oberdick J, Schilling K. The differentiation of cerebellar interneurons is independent of their mitotic history. Neuroscience. 1999;90:1243–1254. - PubMed
-
- Bayer SA, Altman J, Chaper I. San Diego: Academic; 2004. Development in the rat nervous system.
-
- Bermingham JR, Scherer SS, O'Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
