Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer
- PMID: 17507610
- PMCID: PMC4516164
- DOI: 10.1158/1055-9965.EPI-06-0779
Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer
Abstract
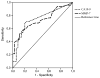
Differentiating between periampullary carcinoma and chronic pancreatitis with an inflammatory mass is difficult. Consequently, 6% to 9% of pancreatic resections for suspected carcinoma are done inappropriately for chronic pancreatitis. Here, we test if matrix metalloproteinase 7 (MMP-7), a secreted protease frequently expressed in pancreatic carcinoma, can be measured in plasma, pancreatic, and duodenal juice, and if it can distinguish between periampullary carcinoma and chronic pancreatitis. Ninety-four patients who underwent pancreatic surgery for a (peri)pancreatic neoplasm (n = 63) or chronic pancreatitis (n = 31) were analyzed. Median plasma MMP-7 levels were significantly higher in carcinoma (1.95 ng/mL; interquartile range, 0.81-3.22 ng/mL) compared with chronic pancreatitis and benign disease (0.83 ng/mL; interquartile range, 0.25-1.21 ng/mL; P < 0.01). MMP-7 levels in pancreatic juice were higher, although not significantly, in carcinoma (62 ng/mg protein; interquartile range, 18-241 ng/mg protein) compared with chronic pancreatitis and benign disease (23 ng/mg protein; interquartile range, 8.5-99 ng/mg protein; P = 0.17). MMP-7 levels in duodenal juice were universally low. At an arbitrary cutoff of 1.5 ng/mL in plasma, positive and negative predictive values were 83% and 57%, respectively, values comparable to those of today's most common pancreatic tumor marker, carbohydrate antigen 19-9 (CA19-9; 83% and 53%, respectively). Positive and negative likelihood ratios for plasma MMP-7 were 3.35 and 0.52, respectively. The area under the receiver operating characteristic curve for MMP-7 was 0.73 (95% confidence interval, 0.63-0.84) and for CA19-9, 0.75 (95% confidence interval, 0.64-0.85). Combined MMP-7 and CA19-9 assessment gave a positive predictive value of 100%. Thus, plasma MMP-7 levels discriminated between patients with carcinoma and those with chronic pancreatitis or benign disease. The diagnostic accuracy of plasma MMP-7 alone is not sufficient to determine treatment strategy in patients with a periampullary mass, but combined evaluation of plasma MMP-7 with CA19-9 and other markers may be clinically useful.
Figures

Similar articles
-
Carbohydrate antigen 19-9 for differential diagnosis of pancreatic carcinoma and chronic pancreatitis.World J Gastroenterol. 2015 Apr 14;21(14):4323-33. doi: 10.3748/wjg.v21.i14.4323. World J Gastroenterol. 2015. PMID: 25892884 Free PMC article. Review.
-
The assessment of serum and diagnostic peritoneal lavage concentration of matrix metalloproteinase-2, matrix metalloproteinase-9, carbohydrate antigen 19-9, and carcinoembryonic antigen in patients with pancreatic cancer and chronic pancreatitis.J Physiol Pharmacol. 2020 Oct;71(5). doi: 10.26402/jpp.2020.5.09. Epub 2021 Feb 8. J Physiol Pharmacol. 2020. PMID: 33571962
-
Serum matrix metalloproteinase-7, Syndecan-1, and CA 19-9 as a biomarker panel for diagnosis of pancreatic ductal adenocarcinoma.Cancer Med. 2024 Sep;13(17):e70144. doi: 10.1002/cam4.70144. Cancer Med. 2024. PMID: 39263943 Free PMC article.
-
Neutrophil Gelatinase-Associated Lipocalin (NGAL) concentration in urine is superior to CA19-9 and Ca 125 in differentiation of pancreatic mass: Preliminary report.Cancer Biomark. 2016 Mar 11;16(4):537-43. doi: 10.3233/CBM-160595. Cancer Biomark. 2016. PMID: 27002756
-
Comparison of circulating MMP-9, TIMP-1 and CA19-9 in the detection of pancreatic cancer.Anticancer Res. 2010 Feb;30(2):587-92. Anticancer Res. 2010. PMID: 20332475
Cited by
-
Matrix-metalloproteinases as targets for controlled delivery in cancer: An analysis of upregulation and expression.J Control Release. 2017 Aug 10;259:62-75. doi: 10.1016/j.jconrel.2017.01.034. Epub 2017 Jan 31. J Control Release. 2017. PMID: 28153760 Free PMC article. Review.
-
Selected Matrix Metalloproteinases (MMP-2, MMP-7) and Their Inhibitor (TIMP-2) in Adult and Pediatric Cancer.Diagnostics (Basel). 2020 Jul 31;10(8):547. doi: 10.3390/diagnostics10080547. Diagnostics (Basel). 2020. PMID: 32751899 Free PMC article. Review.
-
Matrix Metalloproteases in Pancreatic Ductal Adenocarcinoma: Key Drivers of Disease Progression?Biology (Basel). 2020 Apr 18;9(4):80. doi: 10.3390/biology9040080. Biology (Basel). 2020. PMID: 32325664 Free PMC article. Review.
-
Potential usefulness of apolipoprotein A2 isoforms for screening and risk stratification of pancreatic cancer.Biomark Med. 2016 Nov;10(11):1197-1207. doi: 10.2217/bmm-2016-0209. Epub 2016 Sep 27. Biomark Med. 2016. PMID: 27673558 Free PMC article. Review.
-
Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket?World J Gastroenterol. 2021 Jul 14;27(26):4045-4087. doi: 10.3748/wjg.v27.i26.4045. World J Gastroenterol. 2021. PMID: 34326612 Free PMC article. Review.
References
-
- Abraham SC, Wilentz RE, Yeo CJ, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all “chronic pancreatitis”? Am J Surg Pathol. 2003;27:110–20. - PubMed
-
- van Gulik TM, Reeders JW, Bosma A, et al. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–23. - PubMed
-
- van Gulik TM, Moojen TM, van Geenen R, Rauws EA, Obertop H, Gouma DJ. Differential diagnosis of focal pancreatitis and pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):85–8. - PubMed
-
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. - PubMed
-
- Izbicki JR, Bloechle C, Broering DC, Knoefel WT, Kuechler T, Broelsch CE. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998;228:771–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

