Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment
- PMID: 17507648
- PMCID: PMC1949353
- DOI: 10.1091/mbc.e07-02-0166
Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment
Abstract
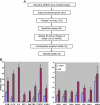
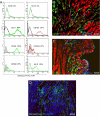
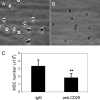
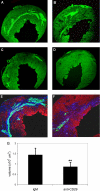
Recent evidence has demonstrated the importance of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the repair of damaged myocardium. The molecular mechanisms of engraftment and migration of BM-MSCs in the ischemic myocardium are unknown. In this study, we developed a functional genomics approach toward the identification of mediators of engraftment and migration of BM-MSCs within the ischemic myocardium. Our strategy involves microarray profiling (>22,000 probes) of ischemic hearts, complemented by reverse transcription-polymerase chain reaction and fluorescence-activated cell sorting of corresponding adhesion molecule and cytokine receptors in BM-MSCs to focus on the coexpressed pairs only. Our data revealed nine complementary adhesion molecules and cytokine receptors, including integrin beta1, integrin alpha4, and CXC chemokine receptor 4 (CXCR4). To examine their functional contributions, we first blocked selectively these receptors by preincubation of BM-MSCs with specific neutralizing antibodies, and then we administered these cells intramyocardially. A significant reduction in the total number of BM-MSC in the infarcted myocardium was observed after integrin beta1 blockade but not integrin alpha4 or CXCR4 blockade. The latter observation is distinctively different from that reported for hematopoietic stem cells (HSCs). Thus, our data show that BM-MSCs use a different pathway from HSCs for intramyocardial trafficking and engraftment.
Figures


References
-
- Abbott J. D., Huang Y., Liu D., Hickey R., Krause D. S., Giordano F. J. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. - PubMed
-
- Anversa P., Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature. 2002;415:240–243. - PubMed
-
- Askari A. T., et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. - PubMed
-
- Assmus B., et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

